��Ŀ����
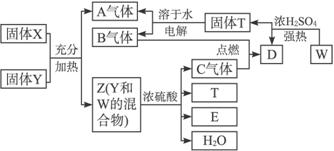
��һ�������¿�ʵ��ͼ��ʾ����֮��ı仯��

����д���¿հף�
��1����ȸʯ����Ҫ�ɷ���Cu2��OH��2CO3�������ֽ⣬��ͼ�е�F�ǣ�д��ѧʽ��
 ��
��
��2��д��������Һ�����NaOH��Һ��Ӧ�����ӷ���ʽ��
��3��ͼ������G��D��Ϊ���壬��Ϻ��ڸ����¿ɷ�����Ӧ��д���÷�Ӧ�ķ�Ӧ����ʽ��
��4��ÿ����1��D��ͬʱ����
��E��
��5��D��NaOH��Һ��Ӧ�Ļ�ѧ��Ӧ����ʽΪ���õ���ʽ�������ת�Ƶķ�����Ŀ��
 ��
��

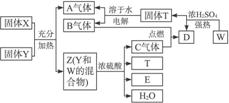
����д���¿հף�
��1����ȸʯ����Ҫ�ɷ���Cu2��OH��2CO3�������ֽ⣬��ͼ�е�F�ǣ�д��ѧʽ��
CO2
CO2
������Ӧ�ĵ���ʽ��

��2��д��������Һ�����NaOH��Һ��Ӧ�����ӷ���ʽ��
Al3++4OH-=AlO2-+2H2O
Al3++4OH-=AlO2-+2H2O
����3��ͼ������G��D��Ϊ���壬��Ϻ��ڸ����¿ɷ�����Ӧ��д���÷�Ӧ�ķ�Ӧ����ʽ��
3CuO+2Al
3Cu+Al2O3
| ||
3CuO+2Al
3Cu+Al2O3
��
| ||
��4��ÿ����1��D��ͬʱ����
| 3 |
| 4 |
| 3 |
| 4 |
��5��D��NaOH��Һ��Ӧ�Ļ�ѧ��Ӧ����ʽΪ���õ���ʽ�������ת�Ƶķ�����Ŀ��


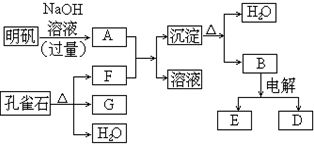
���������ݲ����ý���̼���������ֽ�ɽ����������CO2���Լ������ý������������������ֽ�ɽ����������H2O�Ĺ��ɣ�����֪��ȸʯ�ķֽ����ΪCuO��CO2��H2O��������֪GΪ���弴CuO����FΪ����CO2����������������Լ�Al3+�����ʿ�֪AΪ����NaAlO2����Һ��BΪ�����������ȷֽ�IJ���֮һ��ΪAl2O3�����ݵ��ұ�������Եõ�����������֪DΪAl��EΪO2����Al����ԭ�����Ի�ԭCuO����϶�Ӧ���ʵ����ʺ���ĿҪ��ɽ����⣮
����⣺���ݲ����ý���̼���������ֽ�ɽ����������CO2���Լ������ý������������������ֽ�ɽ����������H2O�Ĺ��ɣ�����֪��ȸʯ�ķֽ����ΪCuO��CO2��H2O��������֪GΪ���弴CuO����FΪ����CO2����������������Լ�Al3+�����ʿ�֪AΪ����NaAlO2����Һ��BΪ�����������ȷֽ�IJ���֮һ��ΪAl2O3�����ݵ��ұ�������Եõ�����������֪DΪAl��EΪO2����Al����ԭ�����Ի�ԭCuO��
��1�������Ϸ�����֪FΪCO2��Ϊ���ۻ��������ʽΪ ���ʴ�Ϊ��CO2��
���ʴ�Ϊ��CO2�� ��
��
��2��������Һ�����NaOH��Һ��Ӧ��ʵ���ǣ������Ӻ�������֮��ķ�Ӧ�����ӷ���ʽΪ��Al3++4OH-=AlO2-+2H2O��
�ʴ�Ϊ��Al3++4OH-=AlO2-+2H2O��
��3��GΪ����ͭ��BΪ�����������ȷֽ�IJ���֮һ��Ϊ��������������Ϊ������������ͭ�����µķ�ӦΪ��3CuO+2Al
3Cu+Al2O3���ʴ�Ϊ��3CuO+2Al
3Cu+Al2O3��
��4�����������ķ�ӦΪ��2Al2O3
4Al+3O2��������������ԭ��Ӧ��ͼ������G��D��Ϊ���壬����DΪ����ÿ����1Ħ����ͬʱ����
mol���������ʴ�Ϊ��
��
��5��Al��NaOH��Ӧ�ķ���ʽΪ2Al+2NaOH+6H2O=2NaAlO2+3H2��+4H2O����Ӧ��Al���ϼ���0�����ߵ�+3�ۣ�ÿmolAlʧȥ3mol����ת�Ƶ�H�������ת�Ƶķ������ĿΪ ��
��
�ʴ�Ϊ�� ��
��
��1�������Ϸ�����֪FΪCO2��Ϊ���ۻ��������ʽΪ
 ���ʴ�Ϊ��CO2��
���ʴ�Ϊ��CO2�� ��
����2��������Һ�����NaOH��Һ��Ӧ��ʵ���ǣ������Ӻ�������֮��ķ�Ӧ�����ӷ���ʽΪ��Al3++4OH-=AlO2-+2H2O��
�ʴ�Ϊ��Al3++4OH-=AlO2-+2H2O��
��3��GΪ����ͭ��BΪ�����������ȷֽ�IJ���֮һ��Ϊ��������������Ϊ������������ͭ�����µķ�ӦΪ��3CuO+2Al
| ||
| ||
��4�����������ķ�ӦΪ��2Al2O3
| ||
| 3 |
| 4 |
| 3 |
| 4 |
��5��Al��NaOH��Ӧ�ķ���ʽΪ2Al+2NaOH+6H2O=2NaAlO2+3H2��+4H2O����Ӧ��Al���ϼ���0�����ߵ�+3�ۣ�ÿmolAlʧȥ3mol����ת�Ƶ�H�������ת�Ƶķ������ĿΪ
 ��
���ʴ�Ϊ��
 ��
�����������⿼��������ƶϣ���Ŀ�Ѷ��еȣ�ע�������ͼ�ʽ̼��ͭ����ɺ����ʣ�Ϊ������Ĺؼ������⿼����ص�Ϊ��ط�Ӧ�ķ���ʽ����д��ע�������д������Ϊ������Ŀ��Ƶ���㣮

��ϰ��ϵ�д�
�����Ŀ