��Ŀ����
����Ŀ��Ħ��������ָ��λ���ʵ��������������е���������֪NAΪ�����ӵ�������ֵ����ش��������⣺
��1����֪NH3����Է�������Ϊ17����NH3��Ħ������Ϊ________��
��2����֪һ����ԭ�ӵ�����Ϊb g������ԭ�ӵ�Ħ������Ϊ________��
��3����֪a gij�����к�������Ϊb����������Ħ������Ϊ________��
��4���������������ʣ���22 g������̼����8 g��������1.204��1024���������� ��4 ��ʱ18 mLˮ����������������������__________������������________������������������________(�����)��
���𰸡�17 g/mol bNA g/mol ![]() g/mol �� �� ��
g/mol �� �� ��
��������
��1������Ħ����������ֵ�ϵ��ڸ����ʵ���Է���������������
��2������Ħ��������1mol���������е�������������
��3������n=![]() �����������ʵ������ٸ���M=
�����������ʵ������ٸ���M=![]() ��������Ħ��������
��������Ħ��������
��4������n=![]() ���������̼�����������ʵ���������n=
���������̼�����������ʵ���������n=![]() ���㰱�������ʵ���������m=��V����ˮ���������ٸ���n=
���㰱�������ʵ���������m=��V����ˮ���������ٸ���n=![]() ����ˮ�����ʵ�����
����ˮ�����ʵ�����
���ʵ���Խ���еķ�����ĿԽ�ࣻ����m=cM���㰱��������������m=��V����ˮ���������ݴ˼���Ƚϣ���ϸ����ʷ��Ӻ��еĵ�����Ŀ������ӵ����ʵ������������ʵ���Խ���е�����ĿԽ�ࡣ
��1��Ħ����������ֵ�ϵ��ڸ����ʵ���Է�����������NH3��Ħ������Ϊ17g/mol��
�ʴ�Ϊ��17g/mol��
��2��Ħ��������1mol���������е�������һ����ԭ�ӵ�����Ϊb g��1mol���к�NA����ԭ�ӣ�����ΪbNA g��������Ħ������ΪbNA g/mol��
�ʴ�Ϊ��bNA g/mol��
��3��a g��������ʵ���Ϊ![]() mol��
mol��
�������Ħ������Ϊ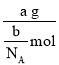 =
=![]() g/mol��
g/mol��
�ʴ�Ϊ��![]() g/mol��
g/mol��
��4����22g������̼�������ʵ���Ϊ![]() =0.5mol��������Ϊ��0.5mol
=0.5mol����������0.5mol![]() 44g/mol=22g��
44g/mol=22g��
��8g�����������ʵ���Ϊ![]() =4mol��
=4mol��
��1.204��1024���������ӣ������ʵ���Ϊ![]() =2mol������Ϊ2mol��28g/mol=56g��
=2mol������Ϊ2mol��28g/mol=56g��
��4��ʱ18mLˮ��������Ϊ18mL��1g/mL=18g�����ʵ���Ϊ![]() =1mol��
=1mol��
�����������������Ǣڣ�
�����������֪����������Ǣۣ�
��22g������̼�����е������ʵ���Ϊ0.5mol��22=11mol��
��8g���������е������ʵ���Ϊ4mol��2=8mol��
��1.204��1024���������ӣ����е������ʵ���Ϊ2mol��14=28mol��
��4��ʱ18mLˮ������ԭ�����ʵ���Ϊ1mol��10=10mol��
�����������������Ǣۣ�
�ʴ�Ϊ���ڣ��ۣ��ۡ�