��Ŀ����
8�� ��N2��g��+3H2��g��?2NH3��g����H��0��Ӧ������������������£��ı���ʼ�������ʵ�������n��H2����ʾ����ʵ��������ͼ1��ʾ��ͼ��T��ʾ�¶ȣ�n��ʾ���ʵ�������
��N2��g��+3H2��g��?2NH3��g����H��0��Ӧ������������������£��ı���ʼ�������ʵ�������n��H2����ʾ����ʵ��������ͼ1��ʾ��ͼ��T��ʾ�¶ȣ�n��ʾ���ʵ���������1���Ƚ���a��b��c����������ƽ��״̬�У���Ӧ��N2��ת������ߵ���c��
��2���������ݻ�Ϊ1L��n=5mol��T2�����·�Ӧ�ﵽƽ��ʱH2��ת����Ϊ60%������H2��N2���ʵ���֮��Ϊ1��1��������ʼʱ��ϵ�м���N2�����ʵ���Ϊ3mol����Ӧ��ƽ�ⳣ��K=1��
���� ��1������ʱ�¶���ͬ��������Խ������ת����Խ��
��2��N2��g��+3H2��g��?2NH3��g��
��ʼ x 5 0
ת�� 1 5��60%=3 2
ƽ��x-1 2 2
H2��N2���ʵ���֮��Ϊ1��1����x-1=2��x=3mol���Դ˼�������ʵ�ƽ��Ũ�ȣ���������ƽ�ⳣ����
��� �⣺��1������ʱ�¶���ͬ��������Խ������ת����Խ����c���ת������ߣ��ʴ�Ϊ��c��
��2��N2��g��+3H2��g��?2NH3��g��
��ʼ x 5 0
ת�� 1 5��60%=3 2
ƽ��x-1 2 2
H2��N2���ʵ���֮��Ϊ1��1����x-1=2��x=3mol��
�����Ϊ1L����ƽ��ʱ�����ʵ�Ũ�ȶ�Ϊ1mol/L��
��K=$\frac{{1}^{2}}{1��{1}^{3}}$=1��
�ʴ�Ϊ��3mol��1��
���� ���⿼�黯ѧƽ����㡢��������Ի�ѧƽ���ƶ�Ӱ�죬Ϊ��Ƶ���㣬���ؿ���ѧ����������������ע������ʽ�������ã���Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
16�� ��1.0L�����ܱ�������Ͷ��1mol CO2��2.75mol H2������Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g����ʵ���ò�ͬ�¶ȼ�ѹǿ�£�ƽ��ʱ�״������ʵ����仯��ͼ��ʾ������˵����ȷ���ǣ�������
��1.0L�����ܱ�������Ͷ��1mol CO2��2.75mol H2������Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g����ʵ���ò�ͬ�¶ȼ�ѹǿ�£�ƽ��ʱ�״������ʵ����仯��ͼ��ʾ������˵����ȷ���ǣ�������
 ��1.0L�����ܱ�������Ͷ��1mol CO2��2.75mol H2������Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g����ʵ���ò�ͬ�¶ȼ�ѹǿ�£�ƽ��ʱ�״������ʵ����仯��ͼ��ʾ������˵����ȷ���ǣ�������
��1.0L�����ܱ�������Ͷ��1mol CO2��2.75mol H2������Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g����ʵ���ò�ͬ�¶ȼ�ѹǿ�£�ƽ��ʱ�״������ʵ����仯��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | �÷�Ӧ������ӦΪ���ȷ�Ӧ | |
| B�� | ѹǿ��С��ϵΪp1��p2��p3 | |
| C�� | M���Ӧ��ƽ�ⳣ��K��ֵԼΪ1.04��10-2 | |
| D�� | ��p2��512 Kʱ��ͼ��N�� v��������v���棩 |
7���������ӷ���ʽ��ȷ���ǣ�������
| A�� | ��̼������Һ��ͨ�������̼��CO32-+CO2+H2O�T2HCO3- | |
| B�� | ����������Ũ���ᷴӦ��MnO2+4HCl��Ũ���TMn2++2Cl2��+2H2O | |
| C�� | ������������Һ�мӹ�������ʯ��ˮ��2HSO3-+Ca2++2OH-�TCaSO3��+2H2O+SO32- | |
| D�� | ������������ͭ��Һ��Ӧ��2Na+2H2O+Cu2+$\frac{\underline{\;\;��\;\;}}{\;}$2Na++Cu��OH��2��+H2�� |




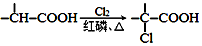

 +NaOH $��_{��}^{ˮ}$
+NaOH $��_{��}^{ˮ}$ +NaCl��
+NaCl�� ��
�� Ϊԭ����ȡ
Ϊԭ����ȡ  �ĺϳ�·��ͼ���ϳ�·��ͼʾ�����£�
�ĺϳ�·��ͼ���ϳ�·��ͼʾ�����£�