��Ŀ����
��13�֣���Ԫ�صĵ��ʺͳ����Ļ������ڹ���ũҵ��������;�㷺��
��1����ҵ�����÷�������ķ����õ���������������Ҫ�ɷֵķе����£�
|
N2 |
O2 |
Ar |
CO2 |
|
��196��C |
��183��C |
��186��C |
��78��C |
�ֽ����������ȴҺ����Ȼ�������£������ȷ�������������� ��
��2������ʱ�����е�N2ת��ΪNO��������NO�� ɫ�����壬 ����ס����ѡ�������ˮ��NO�ڿ����к����ױ�������NO2��NO2����ˮ������ѧ��Ӧ��NO2��ˮ��Ӧ�Ļ�ѧ����ʽΪ ��
��3��ʵ���ҿ��ù���NH4Cl�����Ca(OH)2���ȷ�Ӧ��ȡ������
����ȡ�����Ļ�ѧ����Ϊ ��
��Ҫ��ȡ��״����4.48L�İ�����������Ҫ��ȡ����NH4Cl������Ϊ g��
��4����֪��4NH3+6NO 5N2+6 H2O ����ѧ�о���ѧϰС���ͬѧ�ڼ�����Ա��ָ���£����������̣�̽����ͬ������NH3��ԭNO��Ӧ�Ĵ����ܡ�
5N2+6 H2O ����ѧ�о���ѧϰС���ͬѧ�ڼ�����Ա��ָ���£����������̣�̽����ͬ������NH3��ԭNO��Ӧ�Ĵ����ܡ�
|

����������ʵ����������ͬ���ڴ���Ӧ����װ�ز�ͬ�Ĵ�������������Ӧ��Ļ�����壬ͨ��һ��������з�̪��ϡ������Һ����Һ�������Ũ�Ⱦ���ͬ����
��NH3��ϡ������Һ��Ӧ�����ӷ���ʽΪ ��
��Ϊ�˱Ƚϲ�ͬ�����Ĵ����ܣ���Ҫ��������¼�������� ��
��1��������1�֣� ��2���ޣ��� ������1�֣���2�֣�3NO2+H2O��2HNO3+NO��2�֣�
��3��2NH4Cl+Ca(OH)2 CaCl2+2NH3��+2H2O
��2�� ��
10.7��2�֣�
CaCl2+2NH3��+2H2O
��2�� ��
10.7��2�֣�
��4��NH3 + H+��NH4 + ��Һ��ɫ����Ҫ��ʱ�䣨��2�֣���4�֣�
����������1����������Ҫ�ɷ��ǵ����������������ķе���������ģ����Ե����ȱ����������
��2��NO����ɫ��ζ���ж����壬������ˮ���ڿ����м��ױ��������ɺ���ɫ��NO2��NO2����ˮ������NO��ͬʱ�����������ɣ�����ʽΪ3NO��H2O��2HNO3��NO��
��3��ʵ������ȡ����һ������ʯ�Һ��Ȼ�粒������ɣ�����ʽΪ
2NH4Cl+Ca(OH)2 CaCl2+2NH3��+2H2O����״����4.48L�İ�����
CaCl2+2NH3��+2H2O����״����4.48L�İ�����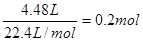 �����Ը��ݷ���ʽ��֪��Ҫ�Ȼ�淋����ʵ���Ҳ��0.2mol��������0.2mol��58.5g/mol��10.7g��
�����Ը��ݷ���ʽ��֪��Ҫ�Ȼ�淋����ʵ���Ҳ��0.2mol��������0.2mol��58.5g/mol��10.7g��
��4���������ڼ������壬����ˮ�Լ��ԣ������ᷴӦ��������李����ӷ���ʽΪNH3 + H+��NH4 +�������Ĵ�Ч���ã���λʱ���ڲ����İ����Ͷ࣬�����ᷴӦʱ����Һ��ɫ�仯����Ҫ��ʱ��ͳ���������Ҫ���������ݾ�����Һ��ɫ����Ҫ��ʱ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д���1����ҵ�����÷�������ķ����õ���������������Ҫ�ɷֵķе����£�
| N2 | O2 | Ar | CO2 |
| -196�� | -183�� | -186�� | -78�� |
��2������ʱ�����е�N2ת��ΪNO��������NO��
��3��ʵ���ҿ��ù���NH4Cl�����Ca��OH��2���ȷ�Ӧ��ȡ������
����ȡ�����Ļ�ѧ����ʽΪ
��Ҫ��ȡ��״����4.48L�İ�����������Ҫ��ȡ����NH4Cl������Ϊ
��4����֪��4NH3+6NO
| ||

����������ʵ����������ͬ���ڴ���Ӧ����װ�ز�ͬ�Ĵ�������������Ӧ��Ļ�����壬ͨ��һ��������з�̪��ϡ������Һ����Һ�������Ũ�Ⱦ���ͬ����
��NH3��ϡ������Һ��Ӧ�����ӷ���ʽΪ
��Ϊ�˱Ƚϲ�ͬ�����Ĵ����ܣ���Ҫ��������¼��������
��13�֣���Ԫ�صĵ��ʺͳ����Ļ������ڹ���ũҵ��������;�㷺��
��1����ҵ�����÷�������ķ����õ���������������Ҫ�ɷֵķе����£�
| N2 | O2 | Ar | CO2 |
| ��196��C | ��183��C | ��186��C | ��78��C |
��2������ʱ�����е�N2ת��ΪNO��������NO�� ɫ�����壬 ����ס����ѡ�������ˮ��NO�ڿ����к����ױ�������NO2��NO2����ˮ������ѧ��Ӧ��NO2��ˮ��Ӧ�Ļ�ѧ����ʽΪ ��
��3��ʵ���ҿ��ù���NH4Cl�����Ca(OH)2���ȷ�Ӧ��ȡ������
����ȡ�����Ļ�ѧ����Ϊ ��
��Ҫ��ȡ��״����4.48L�İ�����������Ҫ��ȡ����NH4Cl������Ϊ g��
��4����֪��4NH3+6NO
 5N2+6 H2O ����ѧ�о���ѧϰС���ͬѧ�ڼ�����Ա��ָ���£����������̣�̽����ͬ������NH3��ԭNO��Ӧ�Ĵ����ܡ�
5N2+6 H2O ����ѧ�о���ѧϰС���ͬѧ�ڼ�����Ա��ָ���£����������̣�̽����ͬ������NH3��ԭNO��Ӧ�Ĵ����ܡ�
|
 ����������ʵ����������ͬ���ڴ���Ӧ����װ�ز�ͬ�Ĵ�������������Ӧ��Ļ�����壬ͨ��һ��������з�̪��ϡ������Һ����Һ�������Ũ�Ⱦ���ͬ����
����������ʵ����������ͬ���ڴ���Ӧ����װ�ز�ͬ�Ĵ�������������Ӧ��Ļ�����壬ͨ��һ��������з�̪��ϡ������Һ����Һ�������Ũ�Ⱦ���ͬ������NH3��ϡ������Һ��Ӧ�����ӷ���ʽΪ ��
��Ϊ�˱Ƚϲ�ͬ�����Ĵ����ܣ���Ҫ��������¼�������� ��
��Ԫ�صĵ��ʺͳ����Ļ������ڹ���ũҵ��������;�㷺��
��1����ҵ�����÷�������ķ����õ���������������Ҫ�ɷֵķе����£�
| N2 | O2 | Ar | CO2 |
| ��196��C | ��183��C | ��186��C | ��78��C |
�ֽ����������ȴҺ����Ȼ�������£������ȷ�������������� ��
��2������ʱ�����е�N2ת��ΪNO��������NO�� ɫ�����壬 ����ס����ѡ�������ˮ��NO�ڿ����к����ױ�������NO2��NO2����ˮ������ѧ��Ӧ��NO2��ˮ��Ӧ�Ļ�ѧ����ʽΪ ��
��3��ʵ���ҿ��ù���NH4Cl�����Ca(OH)2���ȷ�Ӧ��ȡ������
����ȡ�����Ļ�ѧ����ʽΪ ��
��Ҫ��ȡ��״����4.48L�İ�����������Ҫ��ȡ����NH4Cl������Ϊ g��
��4����֪��4NH3+6NO![]() 5N2+6 H2O ����ѧ�о���ѧϰС���ͬѧ�ڼ�����Ա��ָ���£����������̣�̽����ͬ������NH3��ԭNO��Ӧ�Ĵ����ܡ�
5N2+6 H2O ����ѧ�о���ѧϰС���ͬѧ�ڼ�����Ա��ָ���£����������̣�̽����ͬ������NH3��ԭNO��Ӧ�Ĵ����ܡ�
| | |||
����������ʵ����������ͬ���ڴ���Ӧ����װ�ز�ͬ�Ĵ�������������Ӧ��Ļ�����壬ͨ��һ��������з�̪��ϡ������Һ����Һ�������Ũ�Ⱦ���ͬ����
��NH3��ϡ������Һ��Ӧ�����ӷ���ʽΪ ��
��Ϊ�˱Ƚϲ�ͬ�����Ĵ����ܣ���Ҫ��������¼�������� ��
��Ԫ�صĵ��ʺͳ����Ļ������ڹ���ũҵ��������;�㷺��
��1����ҵ�����÷�������ķ����õ���������������Ҫ�ɷֵķе����£�
| N2 | O2[ | Ar | CO2 |
| ��196��C | ��183��C | ��186��C | ��78��C |
�ֽ����������ȴҺ����Ȼ�������£������ȷ�������������� ��
��2������ʱ�����е�N2ת��ΪNO��������NO�� ɫ�����壬 ����ס����ѡ�������ˮ��NO�ڿ����к����ױ�������NO2��NO2����ˮ������ѧ��Ӧ��NO2��ˮ��Ӧ�Ļ�ѧ����ʽΪ ��
��3��ʵ���ҿ��ù���NH4Cl�����Ca(OH)2���ȷ�Ӧ��ȡ������
����ȡ�����Ļ�ѧ����Ϊ ��
��Ҫ��ȡ��״����4.48L�İ�����������Ҫ��ȡ����NH4Cl������Ϊ g��
��4����֪��4NH3+6NO![]() 5N2+6 H2O ����ѧ�о���ѧϰС���ͬѧ�ڼ�����Ա��ָ���£����������̣�̽����ͬ������NH3��ԭNO��Ӧ�Ĵ����ܡ�
5N2+6 H2O ����ѧ�о���ѧϰС���ͬѧ�ڼ�����Ա��ָ���£����������̣�̽����ͬ������NH3��ԭNO��Ӧ�Ĵ����ܡ�

| |
����������ʵ����������ͬ���ڴ���Ӧ����װ�ز�ͬ�Ĵ�������������Ӧ��Ļ�����壬ͨ��һ��������з�̪��ϡ������Һ����Һ�������Ũ�Ⱦ���ͬ����
��NH3��ϡ������Һ��Ӧ�����ӷ���ʽΪ ��
��Ϊ�˱Ƚϲ�ͬ�����Ĵ����ܣ���Ҫ��������¼�������� ��