��Ŀ����
��ѧ�������֮����ת�������������ʵ��������أ�������������������Ҫ��Ӧ�ã�ͬʱҲ��ѧ���γɻ�ѧѧ����������Ҫ��ɲ��֡�
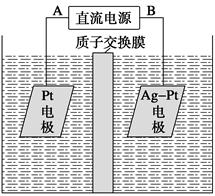
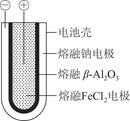
��1������״̬�£��Ƶĵ��ʺ��Ȼ���������ɿɳ���أ���ͼ9��8����ԭ��ʾ��ͼ����Ӧԭ��Ϊ2Na��FeCl2 Fe��2NaCl���õ�طŵ�ʱ��������ӦʽΪ________________________________________________________________________��
Fe��2NaCl���õ�طŵ�ʱ��������ӦʽΪ________________________________________________________________________��
���ʱ��____________��д�������ƣ��缫�ӵ�Դ�ĸ������õ�صĵ����Ϊ________��
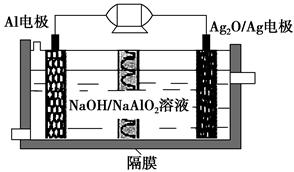
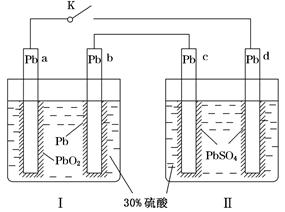
��2��ijͬѧ��ͭƬ��ʯī���缫���һ��Ũ�ȵ�����ͭ��Һ������ԭ��ʾ��ͼ��ͼ��ʾ��һ��ʱ��ֹͣͨ��ȡ���缫�����ڵ������Һ�м���0.98 g������ͭ��ĩǡ����ȫ�ܽ⣬���ⶨ������Һ����ǰ��ȫ��ͬ����ش��������⣺
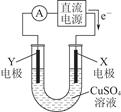
��Y�缫������________������________�����������ԭ������Ӧ��
�ڵ�������X�缫�Ϸ����ĵ缫��Ӧʽ��_______________________________________________________________________��
�����ڵ������Һ�м���������С�մ�ַ�Ӧ����������ڱ�״������ռ�������__________��
��1������״̬�£��Ƶĵ��ʺ��Ȼ���������ɿɳ���أ���ͼ9��8����ԭ��ʾ��ͼ����Ӧԭ��Ϊ2Na��FeCl2
 Fe��2NaCl���õ�طŵ�ʱ��������ӦʽΪ________________________________________________________________________��
Fe��2NaCl���õ�طŵ�ʱ��������ӦʽΪ________________________________________________________________________�����ʱ��____________��д�������ƣ��缫�ӵ�Դ�ĸ������õ�صĵ����Ϊ________��

��2��ijͬѧ��ͭƬ��ʯī���缫���һ��Ũ�ȵ�����ͭ��Һ������ԭ��ʾ��ͼ��ͼ��ʾ��һ��ʱ��ֹͣͨ��ȡ���缫�����ڵ������Һ�м���0.98 g������ͭ��ĩǡ����ȫ�ܽ⣬���ⶨ������Һ����ǰ��ȫ��ͬ����ش��������⣺

��Y�缫������________������________�����������ԭ������Ӧ��
�ڵ�������X�缫�Ϸ����ĵ缫��Ӧʽ��_______________________________________________________________________��
�����ڵ������Һ�м���������С�մ�ַ�Ӧ����������ڱ�״������ռ�������__________��
��1��Fe2����2e��=Fe���ơ��¡�Al2O3
��2����ʯī������
��Cu2����2e��=Cu��2H����2e��=H2����2H2O��2e��=H2����2OH��
��0.448 L��448 mL
��2����ʯī������
��Cu2����2e��=Cu��2H����2e��=H2����2H2O��2e��=H2����2OH��
��0.448 L��448 mL
��1���õ�صĵ����Ϊ���ڵĦ�Al2O3�����ڵ��Ƶ缫���������Ʒ���ʧ���ӵ�������Ӧ�����ڵ�FeCl2�缫��������Fe2�������õ��ӻ�ԭ��Ӧ�����ʱ����ظ����ӵ�Դ��������������ӵ�Դ��������2���ɼ���Cu��OH��2��ʹ�������Һ��ԭ����֪����Ϊʯī������ΪͭƬ���൱�ڶ��Ե缫�������ͭ��Һ������ͭ��������ֵ���ˮ�����������ȷ����ķ�ӦΪCu2����2e��=Cu��Cu2�����ľ����ַ�����Ӧ2H����2e��=H2�������ݵ����غ��ͭԪ�ص��غ㣬��Һ�����ɵ�n��H������2n��Cu2������2��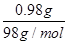 ��0.02 mol��������NaHCO3��Ӧ�ɲ���0.02 mol CO2��
��0.02 mol��������NaHCO3��Ӧ�ɲ���0.02 mol CO2��
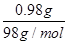 ��0.02 mol��������NaHCO3��Ӧ�ɲ���0.02 mol CO2��
��0.02 mol��������NaHCO3��Ӧ�ɲ���0.02 mol CO2��
��ϰ��ϵ�д�
�����Ŀ

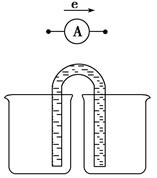
 Fe2����Ag����Ƴ���ͼ��ʾ��ԭ���(����װ����֬���������Һ�����������Ƶ�0�̶Ⱦ��У����Ҿ��п̶�)����֪��ͨ��۲쵽������ָ������ƫת�������ж���ȷ����(����)
Fe2����Ag����Ƴ���ͼ��ʾ��ԭ���(����װ����֬���������Һ�����������Ƶ�0�̶Ⱦ��У����Ҿ��п̶�)����֪��ͨ��۲쵽������ָ������ƫת�������ж���ȷ����(����)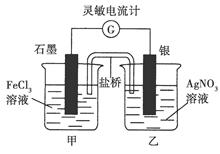
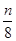 S8
S8 Na2Sn������˵������ȷ����
Na2Sn������˵������ȷ����