��Ŀ����
15��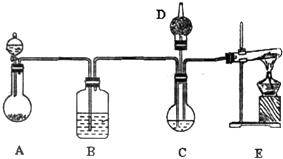 ����̼����й㷺��Ӧ��ǰ��������ͼ��ʾװ�ã���ŨCaCl2��Һ��ͨ��NH3��CO2���Ƶ�����̼��ƣ�D��װ��պϡ�������֬�ޣ�ͼ�мг�װ������ȥ��
����̼����й㷺��Ӧ��ǰ��������ͼ��ʾװ�ã���ŨCaCl2��Һ��ͨ��NH3��CO2���Ƶ�����̼��ƣ�D��װ��պϡ�������֬�ޣ�ͼ�мг�װ������ȥ��I����ѡ�õ�ҩƷ�У�a��ʯ��ʯ b�������Ȼ�����Һ c.6mol•L-1����d���Ȼ�� e����������
��1��A���Ʊ�����ʱ������ҩƷ�ǣ�ѡ����ĸ��ţ�ac��B��ʢ�б���̼��������Һ���������dz�ȥ������̼�е��Ȼ��⣬E�з�����Ӧ�Ļ�ѧ����ʽΪ2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
��2����ʵ������У���C��ͨ�����������Ⱥ�˳��ģ�Ӧ��ͨ������Ļ�ѧʽΪNH3��
��3��д��������̼��ƵĻ�ѧ����ʽCaCl2+CO2+2NH3+H2O=CaCO3��+2NH4Cl��
��4������Ƽ�ʵ�鷽�����ж�����̼�����Ʒ�����Ƿ�Ϊ����������̼��Ƽ�ˮ��ֽ��裬��һ���ɼ������䣬�۲��Ƿ���������������ж����������Ϊ��������û�ж������������������
II���������������Ȼ����Ʒ�к�������̼�����ƣ�Ϊ�˲ⶨ�Ȼ�淋������������������ʵ�����̣�
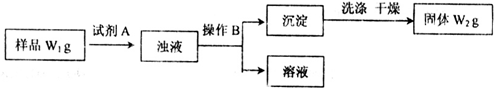
��5�������Լ�A�Ļ�ѧʽΪCa��OH��2��Ba��OH��2������BΪ���ˣ�
��6����Ʒ���Ȼ�淋����������ı���ʽΪ$\frac{{W}_{1}-\frac{84}{100}{W}_{2}}{{W}_{1}}$��100%��$\frac{{W}_{1}-\frac{84}{197}{W}_{2}}{{W}_{1}}$��100%��
���� ��1������װ���ص��֪AΪʯ��ʯ�����ᷴӦ�ƶ�����̼�ķ���װ�ã�EΪ�����ķ���װ�ã���A�ƵõĶ�����̼�к����Ȼ������壬װ��BĿ���dz����ӷ��������Ȼ��⣻ʵ������ȡ���������Ȼ�狀��������Ƽ����Ƶ������Ȼ��ơ�������ˮ��
��2�����ݶ�����̼�Ͱ������ܽ����ж���ͨ������壻
��3���ɷ�Ӧ������������Ԫ���غ�д����Ӧ����ʽ��
��4��������̼��Ƽ�ˮ��ֽ��裬���Ƿ��ж����ЧӦ��
������Ŀ��Ϣ��֪��̼���������������������������Ʒ�Ӧ���ɳ��������ݳ������������̼�����Ƶ��������������Ȼ�淋������Լ�����������
��5��AΪ�����������������ƣ����������Һ�����ù��˵ķ�����
��6�����ݳ������������̼�����Ƶ��������������Ȼ�淋������Լ�����������
��� �⣺��1��װ��AΪ̼��������ᷴӦ�ƶ�����̼������ҩƷ��ʯ��ʯ��6mol/L���ᣬ��ѡac������NaHCO3��Һ�ɳ����ӷ��������Ȼ��⣬ʵ������ȡ���������Ȼ�狀��������Ƽ����Ƶ������Ȼ��ơ�������ˮ������ʽΪ��2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
�ʴ�Ϊ��ac����ȥ������̼�е��Ȼ��⣻2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
��2��������������ˮ�����ں���������̼�����գ�����Ӧ��ͨ�백����
�ʴ�Ϊ��NH3��
��3����������ˮ���ɰ�ˮ����Һ�ʼ��ԣ�������̼���������壬�ܺͼӦ����̼��泥�̼��狀��Ȼ��Ʒ������ֽⷴӦ����̼��ƺ��Ȼ�泥�CaCl2+CO2+2NH3+H2O=CaCO3��+2NH4Cl��
�ʴ�Ϊ��CaCl2+CO2+2NH3+H2O=CaCO3��+2NH4Cl��
��4��������̼��Ƽ�ˮ��ֽ��裬���Ƿ��ж����ЧӦ�����ж����ЧӦ����˵��Ϊ������
�ʴ�Ϊ��������̼��Ƽ�ˮ��ֽ��裬��һ���ɼ������䣬�۲��Ƿ���������������ж����������Ϊ��������û�ж������������������
������Ŀ��Ϣ��֪��̼���������������������������Ʒ�Ӧ���ɳ��������ݳ������������̼�����Ƶ��������������Ȼ�淋������Լ�����������
��5��AΪ�����������������ƣ�̼���������������������������Ʒ�Ӧ���ɳ����������������Һ�����ù��˵ķ�����
�ʴ�Ϊ��Ca��OH��2��Ba��OH��2�����ˣ�
��6�����Լ�ΪCa��OH��2��Һ����
NaHCO3��Ca��OH��2 ��CaCO3
84 100
$\frac{84}{100}$W2 W2
�����Ȼ�淋�����Ϊ��W1-$\frac{84}{100}$W2���Ȼ�淋���������Ϊ��$\frac{{W}_{1}-\frac{84}{100}{W}_{2}}{{W}_{1}}$��100%��
ͬ�������Լ�ΪBa��OH��2��Һ���Ȼ�淋���������Ϊ$\frac{{W}_{1}-\frac{84}{197}{W}_{2}}{{W}_{1}}$��100%��
�ʴ�Ϊ��$\frac{{W}_{1}-\frac{84}{100}{W}_{2}}{{W}_{1}}$��100%��$\frac{{W}_{1}-\frac{84}{197}{W}_{2}}{{W}_{1}}$��100%��
���� ���⿼���˰�����ʵ�����Ʒ��Լ����ʺ����IJⶨ���ѶȲ�������ʵ���ԭ���ǽ���Ĺؼ���

 ��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д�
��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д� Сѧ��ĩ���Ծ�ϵ�д�
Сѧ��ĩ���Ծ�ϵ�д�| A�� | Ϊ����ȡ�������������õĽ������ɽ�����з��� | |
| B�� | ģ��һ��ɷ�Ϊ����ģ�ͺ�˼άģ�� | |
| C�� | ijͬѧ�о�SO2���ʵij����Ƿ��ࣨԤ��SO2�Ļ�ѧ���ʣ����۲죨�ó�SO2���������ʣ���ʵ����Ƚϲ��ó����� | |
| D�� | ��ѧʵ���ܽ����ѧѧ�Ƶ��������� |
| A�� | ԭ��طŵ�ʱ�������Ϸ�����Ӧ��������Zn | |
| B�� | �����Ϸ����ķ�Ӧ��Zn+2OH--2e-�TZn��OH��2 | |
| C�� | ����ʱ����������ҺpH��С��������pH���� | |
| D�� | ��Һ��OH-�������ƶ���K+��H+���ƶ� |


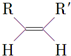 $\stackrel{O_{3}}{��}$$\stackrel{H_{2}O/Zn}{��}$RCHO+R��CHO
$\stackrel{O_{3}}{��}$$\stackrel{H_{2}O/Zn}{��}$RCHO+R��CHO $\stackrel{O_{3}}{��}$$\stackrel{H_{2}O/Zn}{��}$
$\stackrel{O_{3}}{��}$$\stackrel{H_{2}O/Zn}{��}$ ��
�� ��ʵ������ijʵ��С��ͬѧ��������ͭ����ȡ��Ӧ�ã����������ʵ�飺
��ʵ������ijʵ��С��ͬѧ��������ͭ����ȡ��Ӧ�ã����������ʵ�飺
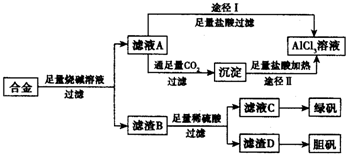 ��ҵ���ú�����������ͭ�ĺϽ�ҵ������ȡ�������Ȼ�����Һ���̷����壨FeSO4•7H2O�� �͵������壨CuSO4•5H2O����������·�����ͼ��ʾ����ش�������⣺
��ҵ���ú�����������ͭ�ĺϽ�ҵ������ȡ�������Ȼ�����Һ���̷����壨FeSO4•7H2O�� �͵������壨CuSO4•5H2O����������·�����ͼ��ʾ����ش�������⣺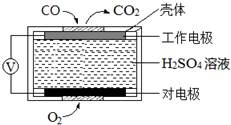 ̼��������������������Ĵ�������������������о����ȵ����⣮
̼��������������������Ĵ�������������������о����ȵ����⣮