��Ŀ����
��ͼ��Ԫ�����ڱ���һ���֣���Ԫ�������ڱ��е�λ�����£��ݴ˻ش��������⣺
��1�����и���Ԫ�����������γ����Ӽ�����
A��c��h B��b��k C��e��j D��e��i
��2��������a��k�γɵĸ������У�����ԭ�Ӷ���������������8���ӽṹ����
A��ga3 B��ak C��hk3 D��ek4
��3�����û�ѧ����ʽ������������
�ٲ�����������ʢ��j���⻯���ˮ��Һ��
��b���������Ӧ��ˮ������Һ����������d��Ʒ�У�
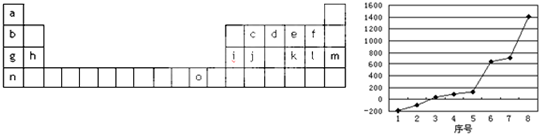
| a | |||||||
| e | g | i | j | ||||
| b | c | d | f | h | k | l | |
B
B
���������γɹ��ۼ�����D
D
A��c��h B��b��k C��e��j D��e��i
��2��������a��k�γɵĸ������У�����ԭ�Ӷ���������������8���ӽṹ����
CD
CD
A��ga3 B��ak C��hk3 D��ek4
��3�����û�ѧ����ʽ������������
�ٲ�����������ʢ��j���⻯���ˮ��Һ��
4HF+SiO2=SiF4��+2H2O
4HF+SiO2=SiF4��+2H2O
��b���������Ӧ��ˮ������Һ����������d��Ʒ�У�
2Al+2NaOH+2H2O=2NaAlO2+3H2��
2Al+2NaOH+2H2O=2NaAlO2+3H2��
����������1�����ý����ͻ��÷ǽ���֮�����γ����Ӽ����ǽ���Ԫ��֮�������γɹ��ۼ���
��2��ԭ�ӵ�����������+|��������Ԫ�ػ��ϼ۾���ֵ|=8ʱ�����Ԫ��ԭ����������������8���ӽṹ��
��3���ٶ��������ܺ�HF�ᷴӦ��
������������Һ�ܺͽ�����������Ӧ��
��2��ԭ�ӵ�����������+|��������Ԫ�ػ��ϼ۾���ֵ|=8ʱ�����Ԫ��ԭ����������������8���ӽṹ��
��3���ٶ��������ܺ�HF�ᷴӦ��
������������Һ�ܺͽ�����������Ӧ��
����⣺����Ԫ�����ڱ��Ľṹ��Ԫ�صķֲ�֪ʶ����֪a��H��b��Na��c��Mg��d��Al��e��C��f��Si��g��N��h��P��i��O��j��F��k��Cl��l��Ar��
��1�����ý���Na�ͻ��÷ǽ���Cl֮�����γ����Ӽ����ǽ���Ԫ��C��O֮���γɵ�CO��CO2�����γɵ��ǹ��ۼ���
�ʴ�Ϊ��B��D��
��2��PCl3��CCl4�и���ԭ�ӵ�����������+|��������Ԫ�ػ��ϼ۾���ֵ|=8������ԭ�Ӿ��ﵽ��8�����ȶ��ṹ���ʴ�Ϊ��CD��
��3���ٲ�������Ҫ�ɷ��Ƕ������裬���������ܺ�HF�ᷴӦ����4HF+SiO2=SiF4��+2H2O�����Բ�����������ʢ��F���⻯���ˮ��Һ���ʴ�Ϊ��4HF+SiO2=SiF4��+2H2O��
��Na���������Ӧ��ˮ������NaOH��Һ������Һ����������Al��Ʒ�У���Ϊ2Al+2NaOH+2H2O=2NaAlO2+3H2�����ʴ�Ϊ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
��1�����ý���Na�ͻ��÷ǽ���Cl֮�����γ����Ӽ����ǽ���Ԫ��C��O֮���γɵ�CO��CO2�����γɵ��ǹ��ۼ���
�ʴ�Ϊ��B��D��
��2��PCl3��CCl4�и���ԭ�ӵ�����������+|��������Ԫ�ػ��ϼ۾���ֵ|=8������ԭ�Ӿ��ﵽ��8�����ȶ��ṹ���ʴ�Ϊ��CD��
��3���ٲ�������Ҫ�ɷ��Ƕ������裬���������ܺ�HF�ᷴӦ����4HF+SiO2=SiF4��+2H2O�����Բ�����������ʢ��F���⻯���ˮ��Һ���ʴ�Ϊ��4HF+SiO2=SiF4��+2H2O��
��Na���������Ӧ��ˮ������NaOH��Һ������Һ����������Al��Ʒ�У���Ϊ2Al+2NaOH+2H2O=2NaAlO2+3H2�����ʴ�Ϊ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
���������⿼��ѧ��Ԫ�����ڱ��Ľṹ�����ʵ�����֪ʶ�����Ը�����ѧ֪ʶ���лش��ѶȲ���

��ϰ��ϵ�д�
�����Ŀ

 �У�BԪ�س�����ۣ�Bԭ��λ����Aԭ����ɵ�����������ģ�������ԭ�ӵ��������Ӿ�����8���ӽṹ�������ӿ��ܾ��еĻ�ѧ������_________��������_______________________________��
�У�BԪ�س�����ۣ�Bԭ��λ����Aԭ����ɵ�����������ģ�������ԭ�ӵ��������Ӿ�����8���ӽṹ�������ӿ��ܾ��еĻ�ѧ������_________��������_______________________________��


 �У�BԪ�س�����ۣ�Bԭ��λ����Aԭ����ɵ�����������ģ�������ԭ�ӵ��������Ӿ�����8���ӽṹ�������ӿ��ܾ��еĻ�ѧ������_________��������_______________________________��
�У�BԪ�س�����ۣ�Bԭ��λ����Aԭ����ɵ�����������ģ�������ԭ�ӵ��������Ӿ�����8���ӽṹ�������ӿ��ܾ��еĻ�ѧ������_________��������_______________________________��