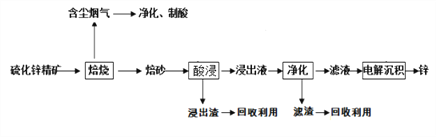
��Ŀ����
����Ŀ������A����Ҫ���л�����ԭ�ϣ���A�����·�Ӧ���Ʊ�һ���л�������
��֪������Ϣ��
�ٺ˴Ź������ױ���Dֻ��һ�ֻ�ѧ�������⣻
���ʻ�������ɷ������·�Ӧ��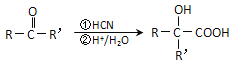 ��ע��R�������������Ҳ������Hԭ�ӣ�
��ע��R�������������Ҳ������Hԭ�ӣ�
��E�ڼ״�������������£�������������ˮ��Ӧ����F��
�ش��������⣺
(1)A�Ľṹ��ʽΪ_____��A����B�ķ�Ӧ����Ϊ_______��
(2)B����C�Ļ�ѧ����ʽΪ_______��
(3)D�Ľṹ��ʽΪ_____������������� __________��ԭ�ӹ�ƽ�档
(4)F�Ļ�ѧ����Ϊ_______��
(5)F��ͬ���칹������ͬʱ�������������Ĺ��� ______�֣����������칹�������к˴Ź���������ʾΪ4��壬�ҷ������Ϊ3 : 2 : 2 : 1����_____�� (д������һ�ֵĽṹ��ʽ����
�����뱥��NaHCO3��Һ��Ӧ�������� ����ʹBr2�����Ȼ�̼��Һ��ɫ
��6�������ᣨ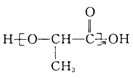 ����һ������ɽ�����ϣ��ο�������Ϣ������Ҵ��Ʊ�������ĺϳ�·�� ___________ ���ϳ�·������ͼͼʾ�����£�
����һ������ɽ�����ϣ��ο�������Ϣ������Ҵ��Ʊ�������ĺϳ�·�� ___________ ���ϳ�·������ͼͼʾ�����£�
![]()
���𰸡� CH2=CHCH3 �ӳɷ�Ӧ ![]() CH3COCH3 6 2-����ϩ����� 8 CH2=C(CH3)CH2COOH��
CH3COCH3 6 2-����ϩ����� 8 CH2=C(CH3)CH2COOH��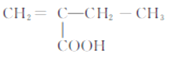
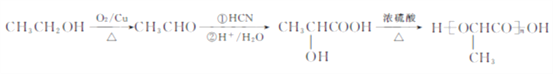
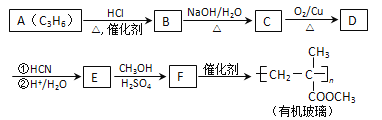
��������F�����Ӿ۷�Ӧ�����л���������F�ṹ��ʽΪCH2=C��CH3��COOCH3��E�ͼ״�����������Ӧ����F��E�ṹ��ʽΪCH2=C��CH3��COOH��A������������A����ʽ֪��A�ṹ��ʽΪCH2=CHCH3��A��HCl�����ӳɷ�Ӧ����B��B����ȡ����Ӧ����C��C������������Ӧ����D���˴Ź������ױ���Dֻ��һ�ֻ�ѧ�������⣬��D�ṹ��ʽΪCH3COCH3��C�ṹ��ʽΪCH3CH��OH��CH3��B�ṹ��ʽΪCH3CHClCH3��D�����ӳɷ�ӦȻ���ữ�õ�E��
��1��A�Ľṹ��ʽΪCH2=CHCH3��A����B�ķ�Ӧ����Ϊ�ӳɷ�Ӧ��
��2��C�ṹ��ʽΪCH3CH��OH��CH3��B�ṹ��ʽΪCH3CHClCH3��B����C�Ļ�ѧ����ʽΪCH3CHClCH3+NaOH![]() CH3CH��OH��CH3+NaCl��
CH3CH��OH��CH3+NaCl��
��3��D�Ľṹ��ʽΪCH3COCH3���ʻ���ƽ��ṹ��HCHOҲ��ƽ��ṹ��CH3COCH3��2����ȡ����ȩ�����е���ԭ�ӣ�ÿ�����������ṩһ����ԭ����֮��ƽ�棬�����������6��ԭ�ӹ�ƽ�棻
��4��F�ṹ��ʽΪCH2=C��CH3��COOCH3��F�Ļ�ѧ����Ϊ2-����ϩ�������
��5��F�ṹ��ʽΪCH2=C��CH3��COOCH3��F��ͬ���칹������ͬʱ��������������
�����뱥��NaHCO3��Һ��Ӧ�������壬˵�������Ȼ���
����ʹBr2�����Ȼ�̼��Һ��ɫ˵������̼̼˫����ȥ���Ȼ��������ĸ�̼ԭ�ӣ������˫�����ĸ�̼��ֱ����2�֣��ֱ����Ȼ�ȡ����Ӧ��6�ֽṹ�������⣻���˫�����ĸ�̼��CH2=C��CH3��2�����Ȼ�ȡ����Ӧ��2�ֽṹ�������⣬����8�ֽṹ�������⣻���к˴Ź���������ʾΪ4��壬�ҷ������Ϊ3�s2�s2�s1����CH2=C��CH3��CH2COOH��
��6�����Ҵ�Ϊ��ʼԭ���Ʊ�������![]() ���Ҵ�������������ȩ����ȩ��HCN�����ӳɷ�Ӧ��Ȼ��ˮ���������ᣬ�����Ӿ۷�Ӧ�����ɾ����ᣬ����Ϊ
���Ҵ�������������ȩ����ȩ��HCN�����ӳɷ�Ӧ��Ȼ��ˮ���������ᣬ�����Ӿ۷�Ӧ�����ɾ����ᣬ����Ϊ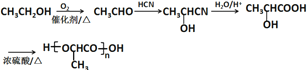 ��
��

����Ŀ��Ϊ�˺������û�ѧ�ܣ�ȷ����ȫ���������������Ҫ��ֿ��ǻ�ѧ��Ӧ�ķ�Ӧ�ȣ�����ȡ��Ӧ��ʩ����ѧ��Ӧ�ķ�Ӧ��ͨ����ʵ����вⶨ��Ҳ�ɽ����������㡣
��1��ʵ���ã�5g�״���CH3OH��Һ���������г��ȼ�����ɶ�����̼�����Һ̬ˮʱ�ͷų�113.5kJ�����������ʾ�״���ȼ���ȵ��Ȼ�ѧ����ʽΪ�� ��
��2���������������Ȼ�ѧ����ʽ����a b����������������������������
H2(g)+ 1/2O2(g)��H2O(g) ��H1��a kJ��mol-1
H2(g)+ 1/2O2(g)��H2O(l) ��H2��b kJ��mol-1
��3����1mol��̬������ij�ֹ��ۼ���Ҫ���յ������м��ܡ��ӻ�ѧ���ĽǶȷ�������ѧ��Ӧ�Ĺ��̾��Ƿ�Ӧ��Ļ�ѧ�����ƻ���������Ļ�ѧ�����γɹ��̡��ڻ�ѧ��Ӧ�����У���ѧ����Ҫ�����������γɻ�ѧ���ֻ��ͷ�������
��ѧ�� | H��H | N��H | N��N |
����/kJ��mol��1 | 436 | 391 | 945 |
��֪��ӦN2(g)��3H2(g)![]() 2NH3(g) ��H��a kJ��mol��1���Ը��ݱ������м������ݹ���a��ֵ��_______________(ע����+����������)��
2NH3(g) ��H��a kJ��mol��1���Ը��ݱ������м������ݹ���a��ֵ��_______________(ע����+����������)��
��4�����ݸ�˹���ɿ��Զ�ijЩ����ͨ��ʵ��ֱ�Ӳⶨ�Ļ�ѧ��Ӧ�ķ�Ӧ�Ƚ������㡣����ˮú���ϳɶ����ѵ�������Ӧ���£�
�� 2H2(g) + CO(g)![]() CH3OH(g)����H ����90.8 kJ��mol��1
CH3OH(g)����H ����90.8 kJ��mol��1
�� 2CH3OH(g)![]() CH3OCH3(g) + H2O(g)����H����23.5 kJ��mol��1
CH3OCH3(g) + H2O(g)����H����23.5 kJ��mol��1
�� CO(g) + H2O(g)![]() CO2(g) + H2(g)����H����41.3 kJ��mol��1
CO2(g) + H2(g)����H����41.3 kJ��mol��1
�ܷ�Ӧ��3H2(g) + 3CO(g)![]() CH3OCH3(g) + CO2(g)����H�� ��
CH3OCH3(g) + CO2(g)����H�� ��
����Ŀ������������һ�ֹ�ҵ�Σ������ʳ�ηdz����ƣ����Խ�ǿ��
��.���飺��Ksp(AgNO2)=2��10��8��Ksp(AgCl)=1.8��10��10����Ka(HNO2)=5.1��10��4���������ķ�������NaNO2��NaCl���ֹ���______________________
��. ijС��ͬѧ������װ�ã���ȥ�г��������Ʊ���������
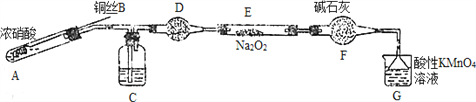
��֪����2NO��Na2O2=2NaNO2�� �����������£�NO��NO2������MnO4����Ӧ����NO3����Mn2����
��1��ʹ��ͭ˿���ŵ���________________________��
��2��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ_____________________________��
װ��C��ʢ�ŵ�ҩƷ��_________��������ĸ���ţ�
A��Ũ���� B��NaOH��Һ C��ˮ D�����Ȼ�̼
��3����С���ȡ5.000g��ȡ����Ʒ����ˮ���250ml��Һ��ȡ25.00ml��Һ����ƿ�У�
��0.1000mol��L��1����KMnO4��Һ���еζ���ʵ�������������±���ʾ��
����� | 1 | 2 | 3 | 4 |
����KMnO4��Һ���/mL | 20.90 | 20.12 | 20.00 | 19.88 |
�ٵ�һ��ʵ�����ݳ����쳣����������쳣��ԭ�������_________������ĸ���ţ���
a����ƿϴ����δ����
b����ʽ�ζ���������ˮϴ����δ�ñ�Һ��ϴ
c���ζ��յ�ʱ���Ӷ���
������KMnO4��Һ�ζ�����������Һ�����ӷ���ʽΪ___________________��
�۸���Ʒ���������Ƶ���������Ϊ______________��