题目内容
氯水的成分及探究
①新制氯水呈__ __色,或氯水能使淀粉碘化钾试纸变蓝,都可以说明___ ____存在
②氯水的PH值__ ___水,说明氯水呈___ _性
③向氯水中滴加紫色石蕊试液产生的现象是_____ ____,可以说明氯水中存在___ ___微粒
④在氯水中加入AgNO3溶液,产生的现象____ _____说明存在__ _____微粒
⑤可以用钢瓶来存在液氯,为了防止钢瓶的腐蚀,冲入氯气前必须_ ____
⑥在氯水中加入无水CuSO4固体,产生的现象____ _____说明存在__ ____微粒
①新制氯水呈__ __色,或氯水能使淀粉碘化钾试纸变蓝,都可以说明___ ____存在
②氯水的PH值__ ___水,说明氯水呈___ _性
③向氯水中滴加紫色石蕊试液产生的现象是_____ ____,可以说明氯水中存在___ ___微粒
④在氯水中加入AgNO3溶液,产生的现象____ _____说明存在__ _____微粒
⑤可以用钢瓶来存在液氯,为了防止钢瓶的腐蚀,冲入氯气前必须_ ____
⑥在氯水中加入无水CuSO4固体,产生的现象____ _____说明存在__ ____微粒
①黄绿色,Cl2 ②小于,酸性③先变红后褪色,H+,HClO ④白色沉淀,Cl- ⑤干燥⑥变蓝,H2O
略

练习册系列答案
相关题目
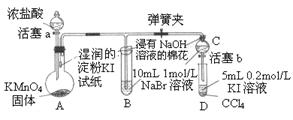
 2和稀盐酸共热来制取氯气
2和稀盐酸共热来制取氯气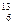 C-NMR(核磁共振)可用于含碳化合物的结构分析。有关
C-NMR(核磁共振)可用于含碳化合物的结构分析。有关