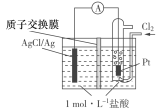
��Ŀ����
����Ŀ������˵������ȷ����
A. ��֪298Kʱ�����ᣨHCN����Ka=4.9��10-10��̼���Ka1=4.4��10-7��Ka2=4.7��10-11���ݴ˿��Ʋ⽫��������뵽̼������Һ�в����ܹ۲쵽�����ݲ���
B. 25��ʱ����amol��L-1��ˮ��0.01mol��L-1����������ϣ���Ӧ��ȫʱ��Һ��c��NH4+��=c��CI-�����ú�a�Ĵ���ʽ��ʾNH3��H2O�ĵ��볣��Kb=![]()
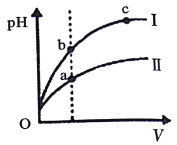
C. ij�¶��£���ͬ�������ͬpH������ʹ�����Һ�ֱ��ˮϡ�ͣ�pH����Һ���V�仯����������ͼ��ʾ��IIΪ����ϡ��ʱpH�ı仯���ߣ���a��b����ˮ�ĵ���̶ȣ�a<b
D. ��0.10mol��L-1NaHSO4��Һ��ͨ��NH3����ҺpH=7��ͨ���������Һ�����Ӱ��ɺ��ԣ���c��Na+��>c��SO42-��>c��NH4+��
���𰸡�D
��������A������H2CO3��HCN��HCO3-��������������뵽̼������Һ�У�û���������ɣ�A��ȷ��B������Һ��c��NH4+��=c��CI-����˵����Һ�����ԣ���c��H+��=c��OH-��=10-7����NH3H2O�ĵ���ƽ�ⳣ��Kb=c(NH4+)��c(OH)/c(NH3H2O)��0.005��107/0.5��(a0.1)��109/(a0.01)��B��ȷ��C������Ϊ���ᣬ���ڵ���ƽ�⣬����ˮϡ��ʱ��������Ũ�ȼ�С���������Դ���ϡ��ʱ��pH����ϻ��������ߢ�Ϊ�����pHֵ�仯������Һ��������Ũ��Խ��ˮ�����Ƴ̶�Խ����a��b����ˮ�ĵ���̶ȣ�a<b��C��ȷ��D�����������غ��֪c��Na+����c��SO42-��>c��NH4+����D����ѡD��