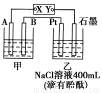
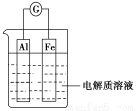
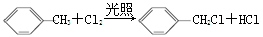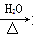ћвƒњƒЏ»Ё
ѕ÷”–A°ҐB°ҐC°ҐD°ҐE°ҐFЅщ÷÷≥£ЉыїѓЇѕќп£ђ“—÷™Ћь√«∞ьЇђµƒ—фјл„””–K£Ђ°ҐAg£Ђ°ҐNa£Ђ°Ґ
Ba2£Ђ°ҐFe2£Ђ°ҐAl3£Ђ£ђ“хјл„””–Cl£≠°ҐOH£≠°ҐAlO2-°ҐNO3-°ҐSO42-°ҐCO32-°£љЂЋь√«Ј÷±р≈д≥…0.1 mol/Lµƒ»№“Їљш––»зѕ¬ µ—й£Ї
Ґў≤вµ√»№“ЇA°ҐC°ҐEЊщ≥ Љо–‘£ђ«“Љо–‘A£ЊE£ЊC£ђEµƒ—ж…Ђ≥ «≥„ѕ…Ђ£®ЌЄєэјґ…Ђо№≤£ЅІєџ≤м£©£їҐЏѕтB»№“Ї÷–µќЉ”ѕ°∞±ЋЃ÷ЅєэЅњ£ђѕ»…ъ≥…≥Ѕµн£ђЇу≥Ѕµн»Ђ≤њ»№љв£їҐџѕтF»№“Ї÷–µќЉ”ѕ°ѕхЋб£ђ»№“Ї±д≥…„Ўї∆…Ђ£ђ«“”–ќё…Ђ∆шће…ъ≥…£їҐ№ѕтD»№“Ї÷–µќЉ”Ba£®NO3£©2»№“Їќё√чѕ‘ѕ÷ѕу°£
£®1£©–і≥цA°ҐD°ҐE°ҐFµƒїѓ—І љ£Ї
A________£їD________£їE________£їF________°£
£®2£©”√јл„”Јљ≥ћ љљв ЌC»№“Ї≥ Љо–‘µƒ‘≠“т£Ї__________________________________°£
£®3£©–і≥ц µ—饟÷–Јі”¶µƒјл„”Јљ≥ћ љ£Ї________________________________°£
£®1£©Ba£®OH£©2 AlCl3 KAlO2 FeSO4
£®2£©CO32-£ЂH2O HCO3-£ЂOH£≠
HCO3-£ЂOH£≠
£®3£©3Fe2£Ђ£ЂNO3-£Ђ4H£Ђ=3Fe3£Ђ£ЂNO°ь£Ђ2H2O
°Њљвќц°њ є»№“Ї≥ Љо–‘µƒ“хјл„””–OH£≠°ҐAlO2-°ҐCO32-£їЄщЊЁ µ—饏њ…“‘»Јґ®Bќ™AgNO3£ђЄщЊЁ µ—饟њ…“‘»Јґ®Fњ…ƒ№ќ™FeCl2їт’яFeSO4£ђЄщЊЁ µ—饹њ…“‘»Јґ®D≤їњ…ƒ№Їђ”–SO42-£ђЋщ“‘Fќ™FeSO4£ђDќ™AlCl3°£”…”Џ«в—хїѓ¬ЅµƒЋб–‘–°”ЏћЉЋб«вЄщµƒ£ђЋщ“‘∆Ђ¬ЅЋб—ќµƒЉо–‘«њ”ЏћЉЋб—ќµƒ£ђљбЇѕЉо–‘A£ЊE£ЊC£ђњ…“‘»Јґ®Aќ™Ba£®OH£©2£ђEќ™KAlO2£ђCќ™Na2CO3£ђC»№“Ї≥ Љо–‘µƒ‘≠“т£ЇCO32-£ЂH2O HCO3-£ЂOH£≠£ђ µ—饟÷–Јі”¶µƒјл„”Јљ≥ћ љќ™3Fe2£Ђ£ЂNO3-£Ђ4H£Ђ=3Fe3£Ђ£ЂNO°ь£Ђ2H2O°£
HCO3-£ЂOH£≠£ђ µ—饟÷–Јі”¶µƒјл„”Јљ≥ћ љќ™3Fe2£Ђ£ЂNO3-£Ђ4H£Ђ=3Fe3£Ђ£ЂNO°ь£Ђ2H2O°£

 ‘ƒґЅњм≥µѕµЅ–ір∞Є
‘ƒґЅњм≥µѕµЅ–ір∞Є