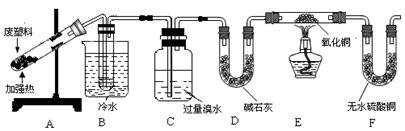
��Ŀ����
ijѧϰС�������������ʵ��̽����ˮ����ɣ�������±��ش����⣮
��1��ָ��ʵ��ٺ�ʵ����е�ʵ������a��______��b��______��
��2����ͬѧ��Ϊʵ��۲�������������Ϊ��ˮ�к��д�������ɵģ����Ƿ�ͬ������۵�______�����ͬ�⡱��ͬ�⡱���������ǣ�______��
��3���ڷ���ʵ������������ԭ��ʱ�����dz����˷��磮��ͬѧ��Ϊ��ˮ���뵽NaOH��Һ�з������кͷ�Ӧ��ʹ��Һ��ɫ����ͬѧ��Ϊ��ˮ�к��д��������Ư���Զ�ʹ��Һ��ɫ��������Ƽ�ʵ��֤�����ǵĹ۵�˭��ȷ��______��
| ʵ����� | ʵ�鷽�� | ʵ������ | ���� |
| �� | ����ˮ�μ�AgNO3��Һ�� | a��______ | ��ˮ�к���Cl- |
| �� | ����ˮ�μӵ�����̪��NaOH��Һ�� | ��ɫ��Һ��Ϊ��ɫ | �� |
| �� | ����ˮ�μӵ�Na2CO3��Һ�� | b��______ | ��ˮ�к���H+ |
��2����ͬѧ��Ϊʵ��۲�������������Ϊ��ˮ�к��д�������ɵģ����Ƿ�ͬ������۵�______�����ͬ�⡱��ͬ�⡱���������ǣ�______��
��3���ڷ���ʵ������������ԭ��ʱ�����dz����˷��磮��ͬѧ��Ϊ��ˮ���뵽NaOH��Һ�з������кͷ�Ӧ��ʹ��Һ��ɫ����ͬѧ��Ϊ��ˮ�к��д��������Ư���Զ�ʹ��Һ��ɫ��������Ƽ�ʵ��֤�����ǵĹ۵�˭��ȷ��______��
��ˮ�д���Cl2+H2O?H++Cl-+HClO����Һ�д���Cl2��HClO��H+��Cl-�����ӣ�
��1������ˮ�μ�AgNO3��Һ�У����а�ɫ�������ɣ���˵����ˮ�к���Cl-������Ag++Cl-=AgCl��������ˮ�μӵ�Na2CO3��Һ�У���Һ�к���H+�������ɶ�����̼���壬������ð����
�ʴ�Ϊ���а�ɫ�������ɣ�������ð����
��2�����������Ա�̼��������̼���Ʋ���Ӧ���ʴ�Ϊ����ͬ�⣻���������Ա�̼���������ܺ�Na2CO3��Ӧ��
��3���кͷ�Ӧ��������ԭ��Ӧ��ԭ����ͬ�������кͷ�Ӧ������ɫ�����Һ�м�NaOH��Һ�����ԣ���Һ�ɱ�Ϊ��ɫ������������ԭ��Ӧ������ɫ�����Һ�м�NaOH��Һ�����ԣ���Һ�����Ժ�ɫ�����ݷ�Ӧ�������֤����
�ʴ�Ϊ������ɫ�����Һ�м�NaOH��Һ�����ԣ���Һ��죬��֤������ȷ�������Ժ�ɫ��֤������ȷ��
��1������ˮ�μ�AgNO3��Һ�У����а�ɫ�������ɣ���˵����ˮ�к���Cl-������Ag++Cl-=AgCl��������ˮ�μӵ�Na2CO3��Һ�У���Һ�к���H+�������ɶ�����̼���壬������ð����
�ʴ�Ϊ���а�ɫ�������ɣ�������ð����
��2�����������Ա�̼��������̼���Ʋ���Ӧ���ʴ�Ϊ����ͬ�⣻���������Ա�̼���������ܺ�Na2CO3��Ӧ��
��3���кͷ�Ӧ��������ԭ��Ӧ��ԭ����ͬ�������кͷ�Ӧ������ɫ�����Һ�м�NaOH��Һ�����ԣ���Һ�ɱ�Ϊ��ɫ������������ԭ��Ӧ������ɫ�����Һ�м�NaOH��Һ�����ԣ���Һ�����Ժ�ɫ�����ݷ�Ӧ�������֤����
�ʴ�Ϊ������ɫ�����Һ�м�NaOH��Һ�����ԣ���Һ��죬��֤������ȷ�������Ժ�ɫ��֤������ȷ��

��ϰ��ϵ�д�
 ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�
�����Ŀ