��Ŀ����
ij�о�С����CaCl2��H2Ϊԭ�ϣ���ͼ�Ʊ� +1��Ca�Ļ����������ֲ�����ֻ�����ֻ�������ң���Ԫ����ɷ���������������иơ���Ԫ�ص����������ֱ�Ϊ52.36%��46.33%���������ҵ�ˮ��Һ�����ԡ���ش��������⣺
��1�����о�С���Ƿ�ɹ��Ƶ� +1��Ca�Ļ���� ����ǡ������Ļ�ѧʽ�� ��
��2������ˮ��Ӧ�ɵ�H2���仯ѧ����ʽ�� ����Ӧ������Һ���ᾧ�ɵõ�һ�־��壬�仯ѧʽΪCaCl2�� xCa(OH)2�� 12H2O��Ϊȷ��x��ֵ�������ʵ�鷽�� ��
��3���ڼ��������£��ҵ�ˮ��Һ��Ũ����MnO2��Ӧ�����ӷ���ʽ�� ���ҵ�ˮ��Һ��Fe��Ӧ���õ���Һ���ȶ����������Һ�Ĵ�ʩ�� ��
��4����д��һ������Ϊ���ܵõ�CaCl�Ļ�ѧ����ʽ����CaCl2Ϊԭ�ϣ� ��
��1�����о�С���Ƿ�ɹ��Ƶ� +1��Ca�Ļ���� ����ǡ������Ļ�ѧʽ�� ��
��2������ˮ��Ӧ�ɵ�H2���仯ѧ����ʽ�� ����Ӧ������Һ���ᾧ�ɵõ�һ�־��壬�仯ѧʽΪCaCl2�� xCa(OH)2�� 12H2O��Ϊȷ��x��ֵ�������ʵ�鷽�� ��
��3���ڼ��������£��ҵ�ˮ��Һ��Ũ����MnO2��Ӧ�����ӷ���ʽ�� ���ҵ�ˮ��Һ��Fe��Ӧ���õ���Һ���ȶ����������Һ�Ĵ�ʩ�� ��
��4����д��һ������Ϊ���ܵõ�CaCl�Ļ�ѧ����ʽ����CaCl2Ϊԭ�ϣ� ��
��1���� ��1�֣�CaHCl ��2�֣�
��2��2CaHCl + 2H2O = CaCl2 + Ca(OH)2 + 2H2����2�֣�
ȡ������ϡHNO3�ܽ��ֳɶ��ȷݣ�����һ�ݼ���Na2CO3��Һ���õ�CaCO3���������غ����n(Ca2+)����һ�ݼ���AgNO3��Һ���õ�AgCl���������غ����n(Cl-)�� ����ʽ�������xֵ��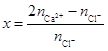 ��3�֣�����������Ҳ���֣�
��3�֣�����������Ҳ���֣�
��3��2Cl- + MnO2 + 4H+�� Mn2+ + Cl2��+ 2H2O ��2�֣�
����FeCl2��Һ�����ԣ����������۷�ֹ���� ��2�֣�
��4��Ca + CaCl2 = 2CaCl
��2��2CaHCl + 2H2O = CaCl2 + Ca(OH)2 + 2H2����2�֣�
ȡ������ϡHNO3�ܽ��ֳɶ��ȷݣ�����һ�ݼ���Na2CO3��Һ���õ�CaCO3���������غ����n(Ca2+)����һ�ݼ���AgNO3��Һ���õ�AgCl���������غ����n(Cl-)�� ����ʽ�������xֵ��
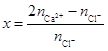 ��3�֣�����������Ҳ���֣�
��3�֣�����������Ҳ���֣���3��2Cl- + MnO2 + 4H+�� Mn2+ + Cl2��+ 2H2O ��2�֣�
����FeCl2��Һ�����ԣ����������۷�ֹ���� ��2�֣�
��4��Ca + CaCl2 = 2CaCl
�����������1����������иơ���Ԫ�ص����������ֱ�Ϊ52.36%��46.33%����������100%С�������������л�������Ԫ�أ�����������Ϊ��100%��52.36%��46.33%��1.31%����ˣ�������ȷ�����������ʵĻ�ѧʽ��
N(Ca)�UN(Cl)�UN(H)��
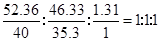
���Եó���ѧʽΪ��CaHCl�����Կ���û�еõ���ν����һ�۵ĸơ�
��2������ˮ��Ӧ�ɵ�H2��˵���Ƿ�������Ԫ�ص�����������ԭ��Ӧ��
2CaHCl + 2H2O = CaCl2 + Ca(OH)2 + 2H2��
ȡ������ϡHNO3�ܽ��ֳɶ��ȷݣ�����һ�ݼ���Na2CO3��Һ���õ�CaCO3���������غ����n(Ca2+)����һ�ݼ���AgNO3��Һ���õ�AgCl���������غ����n(Cl-)�� ����ʽ�������xֵ��
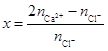
��3���ӵ�һ���п���֪����CaCl2��H2��CaHCl��HCl
2Cl- + MnO2 + 4H+��Mn2+ + Cl2��+ 2H2O
�ҵ�ˮ��Һ��Fe��Ӧ���õ���FeCl2��Һ�����ȶ���һ�����ױ������е��������������Ƕ��������ӻᷢ��ˮ�⣬�������Һ�Ĵ�ʩ�DZ���FeCl2��Һ�����ԣ����������۷�ֹ����
��4��������ǿ�Ļ�ԭ������ԭ����Ca + CaCl2 = 2CaCl

��ϰ��ϵ�д�
 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�
�����Ŀ

 ��100%
��100%