��Ŀ����
1��52gͭþ�Ͻ���ȫ�ܽ���50mL�ܶ�Ϊ1��40g/mL����������Ϊ63%��Ũ�����У��õ�NO2��N2O4�Ļ������1120mL (��״��)����Ӧ�����Һ�м���1��0mol/LNaOH��Һ,����������ȫ������ʱ���õ�2��54g����������˵������ȷ����
| A���úϽ���ͭ��þ�����ʵ���֮����2 �U1 |
| B����Ũ������HNO3�����ʵ���Ũ����14��0mol/L |
| C��NO2��N2O4�Ļ�������У�NO2�����������80% |
| D���õ�2��54����ʱ������NaOH��Һ�������600mL |
D
�������������A��������֪����������ȫ������ʱ���õ�2.54g����Ϊ������ͭ��������þ���ʳ�����������������Ϊ2.54g-1.52g=1.02g�������������ʵ���0.06mol�����ݵ���غ��֪�������ṩ�ĵ������ʵ������������������ʵ�������ͭ��þ�Ͻ���Cu��Mg�����ʵ����ֱ�Ϊxmol��ymol����2x+2y��0.06 64x+24y��1.52�����x=0.02��y=0.01���ʺϽ���ͭ��þ�����ʵ���֮����0.02mol��0.01mol=2��1����ȷ��B����Ũ�����ܶ�Ϊ1.40g/mL����������Ϊ63%���ʸ�Ũ��������ʵ���Ũ��Ϊc=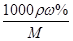 ="1000��1.4��63%/63" =14mol/L����ȷ��C��NO2��N2O4�����������ʵ���Ϊ0.05mol����������������ʵ���Ϊamol�������������������ʵ���Ϊ��0.05-a��mol�����ݵ���ת���غ��֪��a��1+��0.05-a����2��1=0.06�����a=0.04����NO2�����������80%����ȷ��D�������������غ��֪���������Ƶ����ʵ������ڷ�Ӧ����Һ�������Ƶ����ʵ��������ݵ�Ԫ���غ��֪�������Ƶ����ʵ���Ϊ0.05L��14mol/L-0.04mol-��0.05-0.04����2=0.64mol������Ҫ����������Һ�����Ϊ640mL������
="1000��1.4��63%/63" =14mol/L����ȷ��C��NO2��N2O4�����������ʵ���Ϊ0.05mol����������������ʵ���Ϊamol�������������������ʵ���Ϊ��0.05-a��mol�����ݵ���ת���غ��֪��a��1+��0.05-a����2��1=0.06�����a=0.04����NO2�����������80%����ȷ��D�������������غ��֪���������Ƶ����ʵ������ڷ�Ӧ����Һ�������Ƶ����ʵ��������ݵ�Ԫ���غ��֪�������Ƶ����ʵ���Ϊ0.05L��14mol/L-0.04mol-��0.05-0.04����2=0.64mol������Ҫ����������Һ�����Ϊ640mL������
���㣺����������йؼ��㡢�غ�˼����⡣

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д��������ײ�����ȱλ�����Σ�MFe2Ox��3<x<4��M��Mn��Co��Zn��Ni���ڸ����о��ԣ�2�ۣ��������Σ�MFe2O4����������������Ӧ�Ƶã������£�����ʹ��ҵ�����е����������SO2��NO2�ȣ�ת��Ϊ�䵥�ʳ�ȥ��ת��������ͼ�����ڴ�ת�����̵���������ȷ����
| A��MFe2O4����H2�ķ�Ӧ�б����������� |
| B��MFe2O4��MFe2Ox���ת����Ӧ������������ԭ��Ӧ |
| C��MFe2Ox��SO2��Ӧ��MFe2Ox����ԭ |
| D����4 mol MFe2Ox��1 mol SO2ǡ����ȫ��Ӧ����MFe2Ox��x��ֵΪ3��5 |
SCR����ѡ���Դ���ԭ��������һ����NH3��Ϊ��ԭ������������NOx�ֽ������N2��H2O�ĸɷ�������������Ӧԭ��Ϊ����6NO+4NH3��5N2+6H2O����6NO2+8NH3��7N2+12H2O����NO+NO2+2NH3��2N2+3H2O
����˵����ȷ����
| A��NOx��Ҫ����������β�����ŷţ�����������ЧӦ����Ҫ����֮һ |
| B��N2�Цм���Ҽ�֮��Ϊ1:2 |
| C����Ӧ����ÿ����22.4LN2��ת�Ƶ�����1.5NA |
| D��NH3�ķе��PH3�ķе�� |
Ϊ��̽�������仯����������Ժͻ�ԭ�ԣ�ijͬѧ���������ʵ�鷽�������з���ʵ��Ҫ������ȫ��ȷ����
| | ʵ����� | ʵ������ | ���ӷ�Ӧ | ʵ����� |
| A | ���Ȼ�������Һ�еμ�������ˮ | dz��ɫ��Һ����ػ�ɫ��Һ | 2Fe2����Cl2= 2Fe3����2Cl�� | Fe2�����л�ԭ�� |
| B | ���Ȼ�������Һ�м���пƬ | dz��ɫ��Һ�����ɫ��Һ | Fe2����Zn= Fe��Zn2�� | Zn���л�ԭ�� |
| C | ���Ȼ�����Һ�м������� | �ػ�ɫ��Һ���dz��ɫ��Һ | Fe3����Fe= 2Fe2�� | �����ʾ��л�ԭ�� |
| D | ���Ȼ�����Һ�м���ͭ�� | ��ɫ��Һ����ػ�ɫ��Һ | 2Fe3����Cu= 2Fe2����Cu2�� | Fe3������������ |
���ݱ�����Ϣ�жϣ�����ѡ���ȷ����
| ��� | ��Ӧ�� | ���� |
| �� | KMnO4 ��H2O2 ��H2SO4 | K2SO4 ��MnSO4 ������������ |
| �� | Cl2 ��FeBr2 | FeCl3��FeBr3 |
| �� | MnO4�������������� | Cl2 ��Mn2+ ������������ |
A���ڢ��鷴Ӧ���������ΪH2O�� O2
B���ڢ��鷴Ӧ��Cl2 �� FeBr2�����ʵ���֮��С�ڻ����1�U2
C���ڢ��鷴Ӧ������1mol Cl2��ת�Ƶ���2mol
D����������ǿ����˳��ΪMnO4- > Cl2 > Fe3+ > Br2
�ڻ�ҩ������Ʒۡ�����غ�ľ̿��һ��������϶��ɵģ���ըʱ�ķ�Ӧ�ǣ�
S+2KNO3+3C��K2S+N2��+3CO2�����÷�Ӧ�Ļ�ԭ����
| A��C | B��C��S | C��KNO3 | D��S��KNO3 |
����������NF3����һ�����͵��Ӳ��ϣ����ڳ�ʪ�Ŀ�������ˮ�����ܷ���������ԭ��Ӧ���䷴Ӧ�IJ����У�HF��NO��HNO3��������˵����ȷ���ǣ� ��
| A����Ӧ�����У��������뱻��ԭ��Ԫ�ص����ʵ���֮��Ϊ2�U1 |
| B��NF3��һ����ɫ���������壬���NF3�ڿ�����й©ʱ���ױ���� |
| C��һ��NF3й©��������NaOH��Һ���ܵķ������ٿ�����Ⱦ |
| D������Ӧ������1.0mol HNO3��ת�Ƶĵ�����ĿΪ6.02��1023 |
 ="=" 2KCl + 2CrCl3 + 3Cl2+ 7H2O
="=" 2KCl + 2CrCl3 + 3Cl2+ 7H2O