��Ŀ����
(10��)��ͼ��ʾ�����£�U�ι���ʢ��100mL����Һ���밴Ҫ��ش��������⡣
��1������ʢ��ҺΪCuSO4��Һ����K2���պ�K1����
AΪ �����������������B���ĵ缫��ӦʽΪ ��
��2������ʢ��ҺΪ���з�̪��NaCl��Һ����K1���պ�K2����
��A�缫�ɹ۲쵽��������
���ܷ�Ӧ�Ļ�ѧ����ʽ��
�۷�Ӧһ��ʱ����K2 ,��������Һ������仯������
���ܽ⣬B��������������������ɱ�״����Ϊ11.2mL��
����Һ��ֻ�ϣ���Һ��C��OH-��Ϊ
��(10��)��1���� �� �� Cu2++2e- = Cu ��2���� ������������Һ���
�� 2NaCl+2H2Oͨ�� 2NaOH+ H2��+Cl2�� �� 0.01 mol/L ����2�֣�
����:

��ϰ��ϵ�д�
�����Ŀ
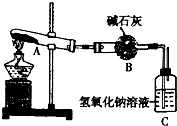 ��2013?֣��һģ��һ���¶��£�����ͭ���ȷ�˲����CuO��SO2��SO3��O2����֪��SO2��SO3���ܱ���ʯ�Һ�����������Һ���գ�������ͼ��ʾװ�ü�������ͭ��ĩֱ����ȫ�ֽ⣮������ͭ��ĩ����Ϊ10.0g����ȫ�ֽ��װ�õ������仯��ϵ�����ʾ�� ��2013?֣��һģ��һ���¶��£�����ͭ���ȷ�˲����CuO��SO2��SO3��O2����֪��SO2��SO3���ܱ���ʯ�Һ�����������Һ���գ�������ͼ��ʾװ�ü�������ͭ��ĩֱ����ȫ�ֽ⣮������ͭ��ĩ����Ϊ10.0g����ȫ�ֽ��װ�õ������仯��ϵ�����ʾ��
|
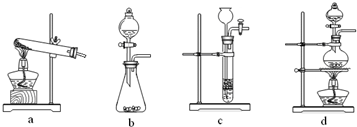

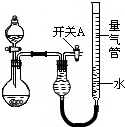 ��2010?���ݶ�ģ��ijͬѧ�����ͼ��ʾװ�÷ֱ����̽��ʵ�飨�г�װ������ȥ��
��2010?���ݶ�ģ��ijͬѧ�����ͼ��ʾװ�÷ֱ����̽��ʵ�飨�г�װ������ȥ��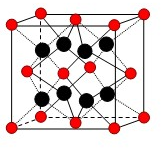 ��U��V��W��X��Y��Z��T����ǰ������Ԫ�أ����ǵ�ԭ���������������������Ϣ���±���
��U��V��W��X��Y��Z��T����ǰ������Ԫ�أ����ǵ�ԭ���������������������Ϣ���±��� 17��
17��