��Ŀ����
��֪�� P4(s)��6Cl2(g) 4PCl3(g) + a kJ�� P4(s)��10Cl2(g)
4PCl3(g) + a kJ�� P4(s)��10Cl2(g) 4PCl5(g) + b kJ��P4������������ṹ��PCl5��P��Cl���ļ���Ϊc kJ/mol��PCl3��P��Cl���ļ���Ϊ1.2c kJ/mol������������ȷ����
4PCl5(g) + b kJ��P4������������ṹ��PCl5��P��Cl���ļ���Ϊc kJ/mol��PCl3��P��Cl���ļ���Ϊ1.2c kJ/mol������������ȷ����
 4PCl3(g) + a kJ�� P4(s)��10Cl2(g)
4PCl3(g) + a kJ�� P4(s)��10Cl2(g) 4PCl5(g) + b kJ��P4������������ṹ��PCl5��P��Cl���ļ���Ϊc kJ/mol��PCl3��P��Cl���ļ���Ϊ1.2c kJ/mol������������ȷ����
4PCl5(g) + b kJ��P4������������ṹ��PCl5��P��Cl���ļ���Ϊc kJ/mol��PCl3��P��Cl���ļ���Ϊ1.2c kJ/mol������������ȷ����| A��P��P���ļ��ܴ���P��Cl���ļ��� |
B������Cl2(g)��PCl3(g) PCl5(s)�ķ�Ӧ�� PCl5(s)�ķ�Ӧ�� |
C��Cl��Cl���ļ���Ϊ 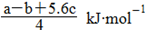 |
D��P��P���ļ���Ϊ  |
C
���������A.������PCl5��P��Cl���ļ���Ϊc kJ/mol����PCl3��P��Cl���ļ���Ϊ1.2c kJ/mol�����ʲ�ͬ�������е�P��Cl���ļ��ܲ�ͬ��������Ƚ�P��P���ļ�����P��Cl���ļ��ܵĴ�С������B.��P4(s)��6Cl2(g)
 4PCl3(g) + a kJ���� P4(s)��10Cl2(g)
4PCl3(g) + a kJ���� P4(s)��10Cl2(g) 4PCl5(g) + b kJ���ڣ��������ɵ�Cl2(g)��PCl3(g)
4PCl5(g) + b kJ���ڣ��������ɵ�Cl2(g)��PCl3(g) PCl5(g) ��
PCl5(g) ��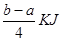 ������û��PCl5(g)
������û��PCl5(g)  PCl5(s)����ЧӦ�����Բ�����Cl2(g)��PCl3(g)
PCl5(s)����ЧӦ�����Բ�����Cl2(g)��PCl3(g) PCl5(s)�ķ�Ӧ�ȡ�����C.���ݷ�Ӧ Cl2(g)��PCl3(g)
PCl5(s)�ķ�Ӧ�ȡ�����C.���ݷ�Ӧ Cl2(g)��PCl3(g) PCl5(g) ��
PCl5(g) ��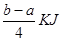 �ķ�Ӧ������ܵĹ�ϵ�ɵ�Cl��Cl��3��1.2c-5��c=
�ķ�Ӧ������ܵĹ�ϵ�ɵ�Cl��Cl��3��1.2c-5��c=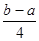 �������ɵ�Cl��Cl�ļ���Ϊ
�������ɵ�Cl��Cl�ļ���Ϊ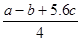 KJ/mol����ȷ��D.���ݼ����뷴Ӧ�ȹ�ϵ ��Cl��Cl�ļ���Ϊ
KJ/mol����ȷ��D.���ݼ����뷴Ӧ�ȹ�ϵ ��Cl��Cl�ļ���Ϊ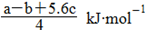 ����ٿɵ�6P��P+6��
����ٿɵ�6P��P+6��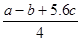 ��4��3��1.2c=a�����P��P���ļ���Ϊ
��4��3��1.2c=a�����P��P���ļ���Ϊ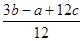 KJ/mol.����
KJ/mol.���� 
��ϰ��ϵ�д�
�����Ŀ
 4NO(g)��CO2(g)��2H2O(g) ��H1��0
4NO(g)��CO2(g)��2H2O(g) ��H1��0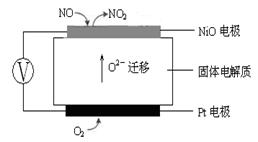

 2CO2(g)+2H2O(l)����H1="-870.3" kJ��mol-1
2CO2(g)+2H2O(l)����H1="-870.3" kJ��mol-1 CO2(g) + H2(g)����Ӧ�����и����ʵ�Ũ������ͼt1ǰ��ʾ�仯���������¶Ȳ��䣬t2ʱ���������г���CO��H2��1mol��ƽ�⽫ �ƶ�������� �����ҡ���������t2ʱ�����ı䷴Ӧ����������H2Ũ�ȷ�������ͼt2����ʾ�ı仯����ı������������ ������ţ���
CO2(g) + H2(g)����Ӧ�����и����ʵ�Ũ������ͼt1ǰ��ʾ�仯���������¶Ȳ��䣬t2ʱ���������г���CO��H2��1mol��ƽ�⽫ �ƶ�������� �����ҡ���������t2ʱ�����ı䷴Ӧ����������H2Ũ�ȷ�������ͼt2����ʾ�ı仯����ı������������ ������ţ���




 CH3CH2OH(g)+3H2O(g) ��H1
CH3CH2OH(g)+3H2O(g) ��H1 H1��
H1��
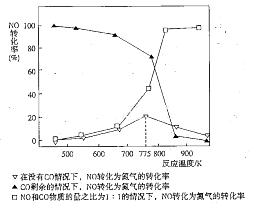
 H2(g)��CO2(g)����H2��
H2(g)��CO2(g)����H2�� CO(g)��H2(g)����H����131.3 kJ��mol��1��
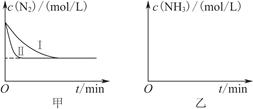
CO(g)��H2(g)����H����131.3 kJ��mol��1��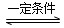 2NH3(g)����H����92.4 kJ��mol��1��ʵ����ģ�⻯���������ֱ��ڲ�ͬʵ�������·�Ӧ��N2Ũ����ʱ��仯��ͼ����ʾ��
2NH3(g)����H����92.4 kJ��mol��1��ʵ����ģ�⻯���������ֱ��ڲ�ͬʵ�������·�Ӧ��N2Ũ����ʱ��仯��ͼ����ʾ��