��Ŀ����
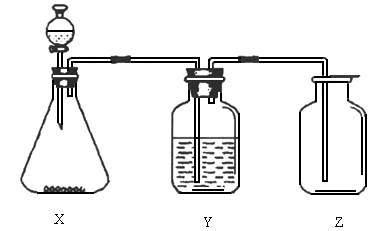
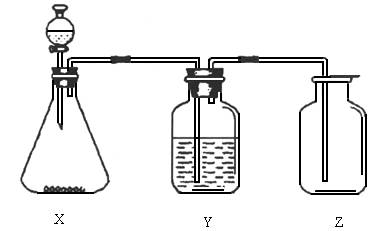
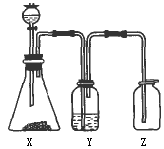
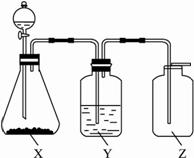
���������������ͼ��ʵ��װ���й���ѡ��A��D�������ո���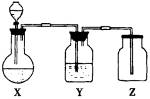
(1)��ȡij��ɫ�ǽ�������������ʱ����ƿX�ڵ�ҩƷӦ��(��)
A��ͭ��ϡHNO3����������������B��ͭ��ŨHNO3
C��CaCO3��ϡH2SO4������������D��Na2SO3��ŨHCl
(2)ϴ��ƿY����װҺ����(��������ˮ���ʻ���������)(��)
A��ŨH2SO4��������������������B��NaOH��Һ
C������NaHSO3����������������D��NaHCO3��Һ
(3)���鼯��ƿ�Ƿ������壬����ƿ�ڵ���ֽӦմ�ϵ���Һ��(��)
A��BaCl2��Һ������������������ B������KMnO4��Һ
C��KI���ۡ���������������������D��ʯ��ˮ
�𰸣�(1)D��(2)C��(3)B

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ?
?