��Ŀ����
��4�֣���֪2NH3(g)  N2(g) +3 H2(g) ����ij�¶��£���2 L�ܱ������м���һ������NH3����Ӧ��10����ʱ����ֵ�Ũ�Ȳ��ٸı䣬��ʱ��ø���ֵ�Ũ�����£�
N2(g) +3 H2(g) ����ij�¶��£���2 L�ܱ������м���һ������NH3����Ӧ��10����ʱ����ֵ�Ũ�Ȳ��ٸı䣬��ʱ��ø���ֵ�Ũ�����£�
��ʱ����ƽ����Ӧ����v(N2) ��
�ﵽƽ��״̬������˵����ȷ���ǣ�
a��ͨ���ı䷴Ӧ ������ʹ����ƽ���ƶ�
������ʹ����ƽ���ƶ�
b��NH3�ķֽ����ʺͺϳ��������
c��NH3���ٷֽ���
d��ͨ���ı䷴Ӧ������ʹNH3ȫ���ֽ��N2��H2
 N2(g) +3 H2(g) ����ij�¶��£���2 L�ܱ������м���һ������NH3����Ӧ��10����ʱ����ֵ�Ũ�Ȳ��ٸı䣬��ʱ��ø���ֵ�Ũ�����£�
N2(g) +3 H2(g) ����ij�¶��£���2 L�ܱ������м���һ������NH3����Ӧ��10����ʱ����ֵ�Ũ�Ȳ��ٸı䣬��ʱ��ø���ֵ�Ũ�����£�| ���� | NH3 | N2 | H2 |
| ���ʵ�����mol�� | 0. 1 | 0.2 | 0.6 |
�ﵽƽ��״̬������˵����ȷ���ǣ�
a��ͨ���ı䷴Ӧ
 ������ʹ����ƽ���ƶ�
������ʹ����ƽ���ƶ�b��NH3�ķֽ����ʺͺϳ��������
c��NH3���ٷֽ���
d��ͨ���ı䷴Ӧ������ʹNH3ȫ���ֽ��N2��H2
0.01moll-1min-1 ab
��

��ϰ��ϵ�д�
�����Ŀ
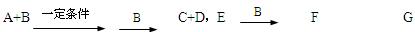
 G�����ӷ���ʽ ��
G�����ӷ���ʽ ��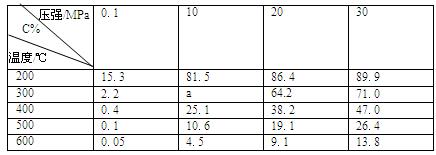

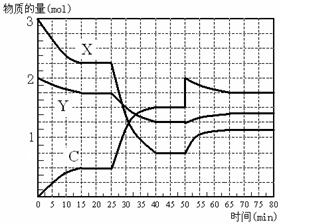
 zC(��)���ﵽƽ��ʱ���A�����Ũ��Ϊ0.5 mol/L�����ں����½��������������һ�����ٴδﵽƽ�⣬���A�����Ũ��Ϊ0.3 mol/L��������������ȷ����
zC(��)���ﵽƽ��ʱ���A�����Ũ��Ϊ0.5 mol/L�����ں����½��������������һ�����ٴδﵽƽ�⣬���A�����Ũ��Ϊ0.3 mol/L��������������ȷ���� 3C(g)����֪����1molA��3molB�Ҵﵽƽ�������amolC���ﵽƽ���C�ڷ�Ӧ������еİٷֺ�����
3C(g)����֪����1molA��3molB�Ҵﵽƽ�������amolC���ﵽƽ���C�ڷ�Ӧ������еİٷֺ����� 2NO
2NO ������һ;��Ϊ ��
������һ;��Ϊ ��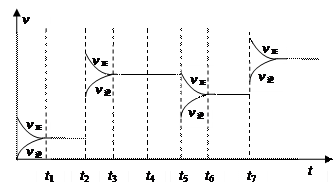
 �����£���������ý��Ϊ���������ոԴ�ѧ�Ļ�ѧ��ʹ����һ����Ϊtrans-Fe(DMeOPrPE)���´������ڳ����ºϳɰ�����Ӧ�Ļ�ѧ����ʽΪ��N2 +3H2
�����£���������ý��Ϊ���������ոԴ�ѧ�Ļ�ѧ��ʹ����һ����Ϊtrans-Fe(DMeOPrPE)���´������ڳ����ºϳɰ�����Ӧ�Ļ�ѧ����ʽΪ��N2 +3H2 2NH3���й�˵����ȷ���� ��
2NH3���й�˵����ȷ���� �� nZ��g��+2W��g��
nZ��g��+2W��g�� 2NH3��g������H��0
2NH3��g������H��0
 Z��g��+2 W��g����H<0����Ӧ���е�5sʱ���X��ת����Ϊ25%��10 s��ﵽ��ѧƽ�⣬���Z��Ũ��Ϊ0.5mol/L��������˵����ȷ����
Z��g��+2 W��g����H<0����Ӧ���е�5sʱ���X��ת����Ϊ25%��10 s��ﵽ��ѧƽ�⣬���Z��Ũ��Ϊ0.5mol/L��������˵����ȷ����