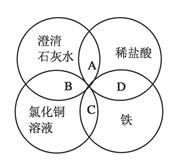
��Ŀ����
ij��ɫ��Һ�к���K+��Cl-��OH-��S ��S
��S ,Ϊ�˼����OH-�����������������,�������ᡢ���ᡢ��������Һ������������Һ����ˮ�ͷ�̪�����Լ�,�������ʵ�鲽��,����¼�������:
,Ϊ�˼����OH-�����������������,�������ᡢ���ᡢ��������Һ������������Һ����ˮ�ͷ�̪�����Լ�,�������ʵ�鲽��,����¼�������: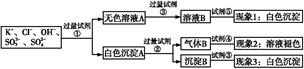
�����йؽ��۴������(����)
| A���Լ�����AgNO3��Һ,�Լ�����HNO3,����1�а�ɫ������AgCl |
| B������3�а�ɫ������BaSO4 |
| C���Լ���������,�Լ��������� |
D����������2�����ӷ���ʽ��:Br2+2H2O+SO2 4H++2Br-+S 4H++2Br-+S |
A
����

��ϰ��ϵ�д�
 ��ѧ�̸̳����¿α�ϵ�д�
��ѧ�̸̳����¿α�ϵ�д� Сѧ��ʱ��ѵϵ�д�
Сѧ��ʱ��ѵϵ�д�
�����Ŀ
���и��������ڳ�����һ���ܴ����������
| A��pH=0����ɫ��Һ�У�Cl����Na+��SO42����Fe2�� |
| B����c(H��)��c(OH��)��1012����Һ�У�NH4����NO3����K����Cl�� |
| C�����������ܷų�H2����Һ�У�Mg2����NH4����NO3����Cl�� |
| D�����д���Fe3+����Һ�У�Al3����SCN����Br����Na+ |
�ס��ҡ�����������������ˮ�����ʣ��ֱ���NH4+��Ba2����Mg2����H����OH���� Cl����HCO3-��SO42-�еIJ�ͬ�����Ӻ������Ӹ�һ����ɣ�������Һ�ֱ��������������ʵ���Һ��ϣ����а�ɫ�������ɣ����Ϊ
| A��MgSO4 | B��Ba(HCO3)2 | C��Mg(HCO3)2 | D��Ba(OH)2 |
�������ӷ���ʽ��д��ȷ����
| A������ʯ��ˮ�������С�մ���Һ��Ӧ��Ca2����OH����HCO3��=CaCO3����H2O |
| B��������Һ�еμ�Ba(OH)2��Һ��SO42�Cǡ����ȫ������Al3++Ba2++ SO42�C+3OH�C=BaSO4��+Al(OH)3�� |
| C��200 mL 2 mol��L�C1��FeBr2��Һ��ͨ��11.2 L��״���µ�������4 Fe2++6Br�C+5Cl2=4Fe3++3Br2+ 10Cl�C |
| D����ǿ����Һ�д���������Fe(OH)3��Ӧ����Na2FeO4��3ClO�C+2Fe(OH)3=2FeO42�C+3Cl�C+H2O+4H+ |
������ط�Ӧ�����ӷ���ʽ��д��ȷ���ǣ� ��
| A������������������Fe(OH)3��3H��=Fe3����3H2O |
| B������ͭ��Һ�����ԣ�Cu2����2H2O=Cu(OH)2����2H�� |
| C����̼�������Һ�мӹ���ʯ��ˮ�����ȣ�NH4+��OH��=NH3����H2O |
| D�����ữ�ĸ��������Һ����˫��ˮ��2MnO4-��6H����5H2O2=2Mn2����5O2����8H2O |
���и�������,һ������ָ�������д����������(����)
| A���ں��д���I-����Һ��:Cl-��Fe3+��Al3+��Cu2+ |
| B������ˮ�������c(H+)=10-12 mol��L-1����Һ��:Na+��Ba2+��Cl-��Br- |
| C����ʹpH��ֽ������Һ��:Fe2+��Na+��SO42����ClO- |
| D���ڼ���Al�ܷų�����H2����Һ��:NH4+��SO42����Cl-��HCO3�� |
�������ӷ���ʽ��ȷ����(����)
| A�����ô����ܽ⺬̼��Ƶ�ˮ����CaCO3��2H��=Ca2����H2O��CO2�� |
B����ͭΪ�缫��ⱥ��ʳ��ˮ��2Cl����2H2O Cl2����H2����2OH�� Cl2����H2����2OH�� |
| C����������Һ�еμӹ�����ˮ��Al3����4NH3��H2O=AlO2����4NH4+��2H2O |
| D����С�մ���Һ�м�����Ba(OH)2��Һ��2HCO3����Ba2����2OH��=BaCO3����2H2O��CO32�� |
���б�ʾ��Ӧ��ѧ��Ӧ�����ӷ���ʽ��ȷ����(����)
A�����Ƶ�ˮ�ⷴӦ��S2����2H2O H2S��2OH�� H2S��2OH�� |
| B������������Һ�еμ��ữ��˫��ˮ��2Fe2����2H����H2O2=2Fe3����2H2O |
| C��̼�������Һ�м��������ռ���Һ��HCO3����OH��=CO32����H2O |
| D�������Լ�ƿ���ռ���Һ��ʴ��SiO2��2Na����2OH��=Na2SiO3����H2O |