��Ŀ����
��10�֣�ijУ��ѧ����С���ѧ��Ϊ����֤�Ҵ��ķ��ӽṹ���������ʵ������Ҵ��ľ��ơ��Ҵ����Ԫ�صIJⶨ������ʽ�IJⶨ�����ӽṹ�IJⶨ��
��1���������Ҵ��к�����ˮ������ֱ������ķ���������Ч��ȥˮ��ͨ�������м��� ��Ȼ������֤���Ҵ����ٺ�ˮ������һ���Լ����飬�����Լ��� ��
��2����ȼ���Ҵ���������ķ���ȷ������C��H��O����Ԫ�ء�
a��֤������HԪ�صIJ����� ��
b��֤������OԪ��ʱ����ȡ�õ�ʵ�������ǣ�CO2��������H2O�������� ��
��3��Ϊȷ���Ҵ��ķ���ʽ����ͨ��(2)��ȡ�Ҵ���ʵ��ʽ֮���Ƿ�����ٲⶨ�Ҵ�����Է�����������ȷ�������ʽ (���ǡ���)�������� ��
��4���ⶨ�Ҵ��ķ��ӽṹ
a�������ú�������Dzⶨ����ͨ�����ú���������� ���������շ壬����ȷ���Ҵ��Ľṹ��CH3CH2OH������CH3OCH3��(�C��H������C��C������C��O������O��H��)
b�������ú˴Ź����Dzⶨ���������Ҵ��ĺ˴Ź���������Ӧ�� �����շ塣
��1���������Ҵ��к�����ˮ������ֱ������ķ���������Ч��ȥˮ��ͨ�������м��� ��Ȼ������֤���Ҵ����ٺ�ˮ������һ���Լ����飬�����Լ��� ��
��2����ȼ���Ҵ���������ķ���ȷ������C��H��O����Ԫ�ء�
a��֤������HԪ�صIJ����� ��
b��֤������OԪ��ʱ����ȡ�õ�ʵ�������ǣ�CO2��������H2O�������� ��
��3��Ϊȷ���Ҵ��ķ���ʽ����ͨ��(2)��ȡ�Ҵ���ʵ��ʽ֮���Ƿ�����ٲⶨ�Ҵ�����Է�����������ȷ�������ʽ (���ǡ���)�������� ��
��4���ⶨ�Ҵ��ķ��ӽṹ
a�������ú�������Dzⶨ����ͨ�����ú���������� ���������շ壬����ȷ���Ҵ��Ľṹ��CH3CH2OH������CH3OCH3��(�C��H������C��C������C��O������O��H��)
b�������ú˴Ź����Dzⶨ���������Ҵ��ĺ˴Ź���������Ӧ�� �����շ塣
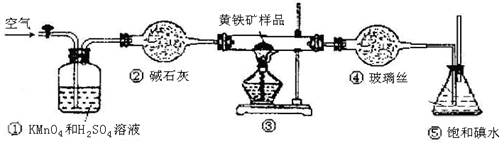
��1��������ʯ�ң�2�֣�����ˮCuSO4��1�֣�4
��2��a ��һ��������С�ձ��������Ҵ�ȼ�ջ�����Ϸ����ձ��ڱ���ˮ�����
�������𰸺���Ҳ���֣���2�֣�
b ������������1�֣�
��3����1�֣���
�Ҵ���ʵ��ʽC2H6O��Hԭ���Ѿ����ͣ�����ʵ��ʽ���������ʽ��1�֣�
��4��a ��O��H����1�֣�
b�� 3��1�֣�
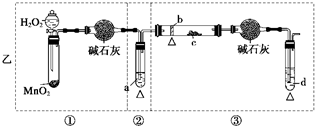
��2��a ��һ��������С�ձ��������Ҵ�ȼ�ջ�����Ϸ����ձ��ڱ���ˮ�����
�������𰸺���Ҳ���֣���2�֣�
b ������������1�֣�
��3����1�֣���
�Ҵ���ʵ��ʽC2H6O��Hԭ���Ѿ����ͣ�����ʵ��ʽ���������ʽ��1�֣�
��4��a ��O��H����1�֣�
b�� 3��1�֣�
��1���Ҵ���ˮ���γɹ����ֱ�������ܵõ���ˮ�Ҵ���һ���Ǽ�����ʯ�ң���������Ҵ���
����ˮ�Ĵ���һ���Dz�����ˮ����ͭ
��2��a�������Ҵ�������Ԫ�أ�ȼ�ղ����а���ˮ��������ˮ����ͭ�����һ��������С�ձ��������Ҵ�ȼ�ջ�����Ϸ����ձ��ڱ���ˮ�����
b��ͨ���Ƚ�CO2��H2O����Ԫ�ص�����֮�ʹ������������������Ҵ��к�����Ԫ��
��3���Ҵ���ʵ��ʽC2H6O��Hԭ���Ѿ����ͣ�����ʵ��ʽ���������ʽ����û�б�Ҫ�ⶨ����Է�������
��4������C2H6O�Ľṹ�������֣�CH3CH2OH��CH3OCH3
a��ǰ���к���O��H����������û�У��ʿ�ͨ������O��H����ȷ����ṹ��ʽ
b��ǰ�߷������������⣬������ֻ��һ����
����ˮ�Ĵ���һ���Dz�����ˮ����ͭ
��2��a�������Ҵ�������Ԫ�أ�ȼ�ղ����а���ˮ��������ˮ����ͭ�����һ��������С�ձ��������Ҵ�ȼ�ջ�����Ϸ����ձ��ڱ���ˮ�����
b��ͨ���Ƚ�CO2��H2O����Ԫ�ص�����֮�ʹ������������������Ҵ��к�����Ԫ��
��3���Ҵ���ʵ��ʽC2H6O��Hԭ���Ѿ����ͣ�����ʵ��ʽ���������ʽ����û�б�Ҫ�ⶨ����Է�������
��4������C2H6O�Ľṹ�������֣�CH3CH2OH��CH3OCH3
a��ǰ���к���O��H����������û�У��ʿ�ͨ������O��H����ȷ����ṹ��ʽ
b��ǰ�߷������������⣬������ֻ��һ����

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ



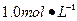 Ba(OH)2��Һ����ֻ�ҵ��ڿ����б�¶�Ѿõ�Ba(OH)2��8H2O�Լ�����ѧʽ����315������������������Һʱ������ȡ�Լ���ˮ�н������ܽ⣬�ձ��д��ڴ���δ���Ϊ̽��ԭ��ͬѧ���Ba(OH)2��8H2O��283K��293K��303Kʱ���ܽ�ȣ�g/100g H2O���ֱ�Ϊ2.5��3.9��5.6��
Ba(OH)2��Һ����ֻ�ҵ��ڿ����б�¶�Ѿõ�Ba(OH)2��8H2O�Լ�����ѧʽ����315������������������Һʱ������ȡ�Լ���ˮ�н������ܽ⣬�ձ��д��ڴ���δ���Ϊ̽��ԭ��ͬѧ���Ba(OH)2��8H2O��283K��293K��303Kʱ���ܽ�ȣ�g/100g H2O���ֱ�Ϊ2.5��3.9��5.6��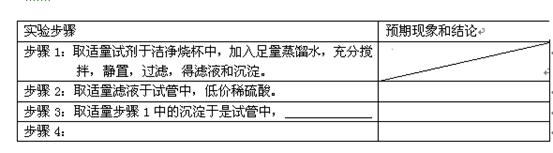
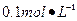 Ba(OH)2��8H2O��Һ��ȷ��ȡw�������������ձ��У�����������ˮ�� ������Һת�� ��ϴ�ӣ����ݣ�ҡ�ȡ�
Ba(OH)2��8H2O��Һ��ȷ��ȡw�������������ձ��У�����������ˮ�� ������Һת�� ��ϴ�ӣ����ݣ�ҡ�ȡ�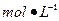 ����װ��50ml��ʽ�ζ��ܣ��ζ����յ㣬��¼���ݡ��ظ��ζ�2�Ρ�ƽ����������Vml��
����װ��50ml��ʽ�ζ��ܣ��ζ����յ㣬��¼���ݡ��ظ��ζ�2�Ρ�ƽ����������Vml��