ћвƒњƒЏ»Ё
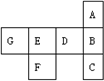
‘™ЋЎa°Ђg‘≠„”µƒ„оЌвµз„”≤гґЉ «M≤г£ђ∆д„оЌв≤гµз„” э»зѕ¬£Ї‘™ЋЎ°°°°°°°°°°°°°°°°°°°° a°°°°°°°°°°°°°°°°°°°° b°°°°°°°°°°°°°°°°°°°° c°°°°°°°°°°°°°°°°°°°° d°°°°°°°°°°°°°°°°°°°° e°°°°°°°°°°°°°°°°°°°° f°°°°°°°°°°°°°°°°°°°° g
„оЌв≤гµз„” э°°°°°°°°°°°°°°°°°°°° 1°°°°°°°°°°°°°°°°°°°° 2°°°°°°°°°°°°°°°°°°°° 3°°°°°°°°°°°°°°°°°°°° 4°°°°°°°°°°°°°°°°°°°° 5°°°°°°°°°°°°°°°°°°°° 6°°°°°°°°°°°°°°°°°°°° 7
”√їѓ—ІЈыЇ≈їЎір£Ї
£®1£©‘≠„”∞лЊґ„о–°µƒ‘™ЋЎ «________£ї
£®2£©‘™ЋЎb”лL≤г…ѕ”–6Єцµз„”µƒ‘™ЋЎљбЇѕ≥…ќ»ґ®їѓЇѕќп£ђ∆дїѓ—І љќ™________£ї
£®3£©„оЄя—хїѓќпµƒЋЃїѓќпЋб–‘„о«њµƒ «________£ђЉо–‘„о«њµƒ «________£ї
£®4£©∆шћђ«вїѓќп„оќ»ґ®µƒ «________£ї
£®5£©‘™ЋЎcµƒ„оЄя—хїѓќпЋЃїѓќп”÷≥∆________–‘«в—хїѓќп£ђЋь”л‘™ЋЎgµƒ«вїѓќпЋЃ»№“ЇЈі”¶µƒїѓ—ІЈљ≥ћ љќ™________________________________£їЋь”л‘™ЋЎaµƒ„оЄя—хїѓќпЋЃїѓќпµƒ»№“ЇЈі”¶њ…±н Њќ™__________________°£
£®6£©a°Ђg‘™ЋЎ÷–£ђ„оЄя—хїѓќпЋЃїѓќп≥ Ћб–‘£ђ«“Ћб–‘”…»хµљ«њµƒЋ≥–т“јіќ «___________
________________________________________°£
љвќц£Ї
| £®1£©Cl £®2£©MgO £®3£©HClO4 NaOH £®4£©HCl
£®5£©Ѕљ Al(OH)3+3HCl®T®TAlCl3+3H2O Al(OH)3+NaOH®T®TNaAlO2+2H2O £®6£©H2SiO3<H3PO4<H2SO4<HClO4
|

 £®2010?¬ђЌе«шґюƒ££©°∞“ќ„”–ќ„і°±µƒЌЉ∞ьЇђЅЋ‘™ЋЎ÷№∆Џ±н«∞Ћƒ÷№∆Џµƒ≤њЈ÷‘™ЋЎ£ђ∆д÷–A°ҐB°ҐC°ҐDЋƒ÷÷‘™ЋЎ‘≠„”–т э÷ЃЇЌќ™77£Ѓ
£®2010?¬ђЌе«шґюƒ££©°∞“ќ„”–ќ„і°±µƒЌЉ∞ьЇђЅЋ‘™ЋЎ÷№∆Џ±н«∞Ћƒ÷№∆Џµƒ≤њЈ÷‘™ЋЎ£ђ∆д÷–A°ҐB°ҐC°ҐDЋƒ÷÷‘™ЋЎ‘≠„”–т э÷ЃЇЌќ™77£Ѓ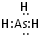
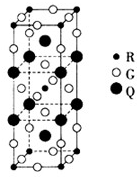 “—÷™A°ҐD°ҐE°ҐG°ҐL°ҐM «ЇЋµзЇ… э“јіќ‘ціуµƒ6÷÷ґћ÷№∆Џ÷ч„е‘™ЋЎ£ђ∆д÷–Aµƒ‘≠„”–т э”л÷№∆Џ–т эѕаµ»£ђD°ҐG°ҐL°ҐMїщћђ‘≠„”µƒ„оЌвƒ№≤гЊщ”–2Єцќі≥…ґ‘µз„”£ЃR+ЇЋЌв”–28Єцµз„”£Ѓ«лїЎірѕ¬Ѕ–ќ ћв£Ї£®ірћв ±£ђA°ҐD°ҐE°ҐG°ҐL°ҐM°ҐR”√Ћщґ‘”¶µƒ‘™ЋЎЈыЇ≈±н Њ£©
“—÷™A°ҐD°ҐE°ҐG°ҐL°ҐM «ЇЋµзЇ… э“јіќ‘ціуµƒ6÷÷ґћ÷№∆Џ÷ч„е‘™ЋЎ£ђ∆д÷–Aµƒ‘≠„”–т э”л÷№∆Џ–т эѕаµ»£ђD°ҐG°ҐL°ҐMїщћђ‘≠„”µƒ„оЌвƒ№≤гЊщ”–2Єцќі≥…ґ‘µз„”£ЃR+ЇЋЌв”–28Єцµз„”£Ѓ«лїЎірѕ¬Ѕ–ќ ћв£Ї£®ірћв ±£ђA°ҐD°ҐE°ҐG°ҐL°ҐM°ҐR”√Ћщґ‘”¶µƒ‘™ЋЎЈыЇ≈±н Њ£©