��Ŀ����
���Ṥҵβ���ж�������ĺ�������0.05%(�������)ʱ���辭����������ŷš�ijУ��ѧ��ȤС�����ⶨij���Ṥ���ŷ�β���ж�������ĺ������ֱ�������·�����
������������ͼ��ʾ��ͼ������������B����ȷ����ͨ����β���������β��ͨ��һ�������֪Ũ�ȵĵ�ˮ�вⶨSO2�ĺ�������ϴ��ƿC����Һ��ɫ��ʧʱ�������رջ���A��
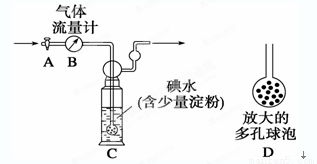
(1)ϴ��ƿC�е���ĩ������һ���������D����Ŀ����______________________________��
(2)ϴ��ƿC�е���Һ�������������Լ����棬�����Ը��������Һ������Ϊѡ�����Ը��������Һ��������________________________________________________________��
(3)ϴ��ƿC����Һ��ɫ��ʧ����û�м�ʱ�رջ���A�����õ�SO2����____________(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)����ijʵ��С��ͬѧ��õ�SO2��������ƫ�ͣ����ܵ�ԭ����__________________________________________________��������ʵ��װ�á�����������ҩƷ��ʵ����������������
���ҷ�������ʵ�鲽������������ͼ��ʾ��
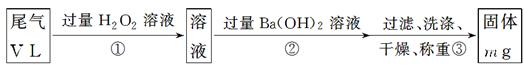
(4)д��������з�Ӧ�Ļ�ѧ����ʽ____________________________________________��
(5)��������жϳ����Ѿ�ϴ�Ӹɾ��ķ�����_______________________________________��
(6)ʵ������ͨ����β�����Ϊ33.6L (�ѻ���ɱ�״��)���������ù�������Ϊ0.233g����ͨ������ȷ����β���ж�������ĺ����Ƿ�ﵽ�ŷű�(д���������)��
��10�֣���1��������������Һ�ĽӴ������������SO2���ˮ��ַ�Ӧ��1�֣�
��2�����Ը��������Һ�ܹ��������SO2����֮��Ӧ����Ӧ����ʱ�������ڹ۲죨�����������𰸣���1�֣� ��3��ƫ�� ͨ��β�����ʹ��죬SO2���ղ���֣���1�֣�
��4��H2SO4+Ba(OH)2=BaSO4��+2H2O��2�֣�
��5��ȡ���һ�ε�ϴ��Һ��������PH��ֽ����PHֵ�����Na2SO4��Һ�����Ƿ��л��dz��֣������������𰸣���2�֣�
��6�������غ��֪n(SO2)=n(BaSO4)="0.001" mol���õ�SO2���������Ϊ0.067%����0.05%��δ��꣨2�֣�
��������
�����������1���������D��������������Һ�ĽӴ������������SO2���ˮ��ַ�Ӧ��
��2����Ϊ���Ը��������Һ����ǿ�����ԣ��ܹ��������SO2����֮��Ӧ�������Ը��������Һ���Ϻ�ɫ����Ӧ����ʱ�������ڹ۲졣
��3��ϴ��ƿC����Һ��ɫ��ʧ����û�м�ʱ�رջ���A������ͨ��������ƫ�࣬��˲�õ�SO2����ƫ�͡���õ�SO2��������ƫ�ͣ����ܵ�ԭ����ͨ��β�����ʹ��죬SO2���ղ��������ġ�
��4��˫��ˮ���������ԣ��ܰ�SO2�����������ᣬ���Է�Ӧ�ڵĻ�ѧ����ʽ��H2SO4+Ba(OH)2=BaSO4��+2H2O��
��5����������жϳ����Ѿ�ϴ�Ӹɾ��ķ��������dz�������Ҳ����ͨ��������Һ��pHֵ����ȡ���һ�ε�ϴ��Һ��������PH��ֽ����PHֵ�����Na2SO4��Һ�����Ƿ��л��dz��֣������������𰸣���
��6���������ù��������ᱵ��������Ϊ0.233g�����ʵ�����0.233g��233g/mol��0.001mol������Ϊβ�������ʵ�����33.6L��22.4L/mol��1.5mol
���Ը�β���ж�������ĺ�����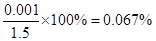
��SO2�������������0.05%����
���㣺����SO2β�����յ��ۺ���ʵ���ж�
�������������е��Ѷȵ����⣬Ҳ�Ǹ߿��еij������㣬�����ۺ���ǿ������������������ʵ�����������ѵ�����ѶȽϴ�ѧ�����÷֡����������ۺ���ǿ�����ۺ�ʵ������ϵ���ܣ��еĻ��ṩһЩ�µ���Ϣ�����Ҫ��ѧ���������桢ϸ�µ����⣬��ϵ��ѧ����֪ʶ�ͼ��ܣ�����֪ʶ����ȡ�Ǩ�ơ����飬ȫ��ϸ�µ�˼�����ܵó���ȷ�Ľ��ۡ�

 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д���1����ҵ����Na2SO3��Һ������ҵβ���е�SO2���������ݱ�ʾ��Ӧ������
| n(SO32-) |
| n(HSO3-) |
|
91��9 | 1��1 | 9��91 | ||
| ������pH | 8.2 | 7.2 | 6.2 |
| n(SO32-) |
| n(HSO3-) |
��2����֪Ki1��H2SO3����Ki��HAc����Ki2��H2SO3����Ki2��H2CO3����ҪʹNaHSO3��Һ��c��Na+����c��HSO3-���ӽ�1��1��������Һ�м�������
a��H2SO3��Һ b��NaOH��Һ c�������� d��Na2CO3
��3��ʵ����ͨ�����µ��KHSO4��Һ�Ʊ����������K2S2O8��д������KHSO4�ĵ��뷽��ʽ��
��4��S2O82-��ǿ�����ԣ���ԭ����ΪSO42-�������̣�MnSO4��������أ�K2S2O8����������Һ�������Ӵ��¿ɷ�����Ӧ���õ��Ϻ�ɫ��Һ����д�˷�Ӧ�Ļ�ѧ����ʽ��
��5����֪��S2O32-�н�ǿ�Ļ�ԭ�ԣ�ʵ���ҿ���I-�ⶨK2S2O8��Ʒ�Ĵ��ȣ���Ӧ����ʽΪ��
S2O82-+2I-��2SO42-+I2 ���٣�
I2+2S2O32-��2I-+S4O62-���ڣ�
��S2O82-��S4O62-��I2������ǿ��˳��
��6��K2S2O8��ƫ����ϩ��CH2=CF2���ۺϵ���������ƫ����ϩ��CH3-CClF2������ȥHCl�Ƶã�����0.5molƫ����ϩ����Ҫ����54kJ���ȣ�д����Ӧ���Ȼ�ѧ����ʽ
��Ԫ�صĺ��������ڹ�ҵ����;�㷺�����������ա�
��ҵ����Na2SO3��Һ������ҵβ���е�SO2���±����ݱ�ʾ��Ӧ������ ��pH�仯�Ĺ�ϵ��
��pH�仯�Ĺ�ϵ��
|
|
91:9 |
1:1 |
9:91 |
|
������pH |
8.2 |
7.2 |
6.2 |
��1������ = 1ʱ����ҺpH= 7.2��ԭ��___________________������0.20 mol/L ��NaOH��Һ����Ӧǰ����Һ������䣩����SO2������Ӧ����Һ�����ԣ���
= 1ʱ����ҺpH= 7.2��ԭ��___________________������0.20 mol/L ��NaOH��Һ����Ӧǰ����Һ������䣩����SO2������Ӧ����Һ�����ԣ���
c (HSO3-) + 2c (SO32-) = _______ mol/L ��
��2����֪��Ki1(H2SO3)> Ki(HAc) > Ki2(H2SO3) > Ki2(H2CO3)��ҪʹNaHSO3��Һ��c(Na+):c(HSO3-)�ӽ�1:1��������Һ�м�������____________��
a��H2SO3��Һ b��NaOH��Һ c�������� d��Na2CO3
��3��ʵ����ͨ�����µ��KHSO4��Һ�Ʊ����������K2S2O8��д������KHSO4�ĵ��뷽��ʽ��__________________________________________��
��4��S2O82-��ǿ�����ԣ���ԭ����ΪSO42-�������̣�MnSO4��������أ�K2S2O8����������Һ�������Ӵ��¿ɷ�����Ӧ���õ��Ϻ�ɫ��Һ����д�˷�Ӧ�Ļ�ѧ����ʽ�� ��
��5����֪��S2O32-�н�ǿ�Ļ�ԭ�ԣ�ʵ���ҿ���I-�ⶨK2S2O8��Ʒ�Ĵ��ȣ���Ӧ����ʽΪ��
S2O82-��2I-��2SO42-��I2 ������ I2��2S2O32-��2I-��S4O62- ������
S2O82-��S4O62-��I2������ǿ��˳��__________________________��
��6��K2S2O8��ƫ����ϩ��CH2=CF2���ۺϵ���������ƫ����ϩ��CH3��CClF2������ȥHCl�Ƶã�����0.5 molƫ����ϩ����Ҫ����54 kJ���ȣ�д����Ӧ���Ȼ�ѧ����ʽ_______��


 Fe��OH��3+3H+ 2H++CaCO3=Ca2++CO2��+H2O
Fe��OH��3+3H+ 2H++CaCO3=Ca2++CO2��+H2O Mn3O4+SO2��+2SO3��+3H2O
Mn3O4+SO2��+2SO3��+3H2O 2SO3(g)����H��0��
2SO3(g)����H��0��