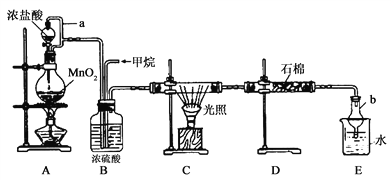
��Ŀ����
����Ŀ����֪ͭ��Ũ��������ڼ��������·������·�Ӧ��
Cu+2H2SO4(Ũ)![]() CuSO4+ SO2 �� +2H2O
CuSO4+ SO2 �� +2H2O
(1)ȡһ������ͭƬ��100mL18mol/L��ŨH2SO4��ַ�Ӧ������÷�Ӧ������ת����0.2mol���ӣ��������ɵ�CuSO4������_______�����ɵ�SO2�����ڱ�״�������_______����������ȫ���ݳ�����
(2)����Ӧ�����õ�����Һ������Ba(OH)2��Һ��ַ�Ӧ�����㣺��Ӧ�����ó���������_______��������0.1g����
���𰸡�16g 2.24L 405.9g
��������
(1)��Ϸ�Ӧ�ķ���ʽ�Լ�������ԭ��Ӧ�е�ʧ������Ŀ��ȼ��㡣
(2)����Ԫ���غ㣬�ȼ����Ũ������S�����ʵ�������ȥSO2�����ʵ������͵õ���Ӧ����Һ��SO42-�����ʵ���������Ba(OH)2��Һ����Ӧ����BaSO4��������Һ�е�Cu2+��OH-����γ�Cu(OH)2��������Ӧ�����ó���ΪCu(OH)2��BaSO4������Ԫ���غ㣬�ɼ����Cu(OH)2��BaSO4�����������͡�
(1)Cu��ŨH2SO4��Ӧ����ʽΪ��Cu+2H2SO4(Ũ)![]() CuSO4+ SO2 �� +2H2O����֪������ת��2mol���ͻ�����1molSO2�����1molCuSO4����÷�Ӧ������ת����0.2mol���ӣ����ɵ�0.1molSO2 ���壬���ڱ�״�������ΪV(SO2)=0.1mol��22.4L/mol=2.24L�����ɵ�CuSO4�����ʵ���Ϊ0.1mol��������Ϊm(CuSO4)=0.1mol��160g/mol=16g��
CuSO4+ SO2 �� +2H2O����֪������ת��2mol���ͻ�����1molSO2�����1molCuSO4����÷�Ӧ������ת����0.2mol���ӣ����ɵ�0.1molSO2 ���壬���ڱ�״�������ΪV(SO2)=0.1mol��22.4L/mol=2.24L�����ɵ�CuSO4�����ʵ���Ϊ0.1mol��������Ϊm(CuSO4)=0.1mol��160g/mol=16g��
(2)n(H2SO4)=cV=18mol/L��0.1L=1.8mol����Ӧ����SO2���壬���ĵ�SO42-�����ʵ�����0.1mol����˷�Ӧ����Һ�к��е�SO42-�����ʵ���Ϊn(SO42-)=1.8mol-0.1mol=1.7mol�������Һ�м�������Ba(OH)2��Һ����Ӧ����BaSO4����������SԪ���غ㣬��֪BaSO4���������ʵ�����1.7mol����m(BaSO4)=1.7mol��233g/mol=396.1g������(1)�����֪n(CuSO4)=0.1mol������Ba(OH)2��Cu2+ת��ΪCu(OH)2�����������CuԪ���غ�ɵ�n[Cu(OH)2]=n(CuSO4)=0.1mol��m[Cu(OH)2]=0.1mol��98g/mol=9.8g���������ɵó���������Ϊ��396.1g+9.8g=405.9g��

 ��������ϵ�д�
��������ϵ�д�����Ŀ��������ƿʧȥ��ǩ����ɫ��Һ��������CaCl2��AgNO3��HCl��Na2CO3�е�����һ�֣�Ϊ��ȷ������ɷ֣����⽫��ƿ��Һ���ΪA��B��C��D��������������ʵ�顣
ʵ��˳�� | ʵ������ | ʵ������ |
�� | A+B | ���������� |
�� | B+D | ������ų� |
�� | B+C | ������� |
�� | A+D | ������� |
���ݱ���ʵ������ش��������⣺
��1��A��B��C��D�ֱ��Ӧ�����ʻ�ѧʽΪ____________��___________��___________��___________��
��2����ֱ�д������ʵ��ں͢۵����ӷ���ʽ��
��________________________________________________________��
��________________________________________________________��