��Ŀ����
��״���£�1���ˮ�����ܽ�500�����HCl���塣����1 Lˮ��ͨ���״���µ�448LHCl���壬����������ȫ�ܽ⡣
��1����������Һ�ܶ�Ϊ1.2 g/cm3������Һ�к�HCl���ʵ���Ũ��Ϊ ��
��2���Ӹ���Һ��ȡ��10mLŨ�����ܽ���ˮ���Ƴ�500mL��Һ�����ƺ��ϡ��Һ�к�HCl���ʵ���Ũ��Ϊ ��
��3������Ũ������������ϡ����ʱ�����������У�ʹ��ǰ�������Ƿ�©Һ�������� �����ƹ����У����Ũ��ƫ�͵IJ���������_______________��ѡ�����в�������ţ���
E������ʱ������Һ���ˮ���̶���
��1����������Һ�ܶ�Ϊ1.2 g/cm3������Һ�к�HCl���ʵ���Ũ��Ϊ ��
��2���Ӹ���Һ��ȡ��10mLŨ�����ܽ���ˮ���Ƴ�500mL��Һ�����ƺ��ϡ��Һ�к�HCl���ʵ���Ũ��Ϊ ��
��3������Ũ������������ϡ����ʱ�����������У�ʹ��ǰ�������Ƿ�©Һ�������� �����ƹ����У����Ũ��ƫ�͵IJ���������_______________��ѡ�����в�������ţ���
| A������ƿ����ˮϴ��δ�Ӹ��� |
| B����Ͳ������ˮϴ��δ���� |
| C�����ձ���Ũ������������ƿ��δ��ˮϴ���ձ������������м�ˮ���̶� |
| D���ý�ͷ�ι�������ƿ�м�ˮʱ�����������̶��ߣ������⽺ͷ�ιܴ�ƿ������������Һʹʣ����Һ���ɴ�̶��� |
��1��13.9 mol/L��δ����λ��1�֣���2�֣�
��2��0.278 mol/L��δ����λ��1�֣���2�֣�
��3������ƿ��������ƿ���ζ��ܣ���2�֣� B C D��3�֣�
��2��0.278 mol/L��δ����λ��1�֣���2�֣�
��3������ƿ��������ƿ���ζ��ܣ���2�֣� B C D��3�֣�
���������
��1��HCl���ʵ���Ũ��Ϊ
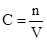 ��448LHClΪ20mol������Һ���Ϊ
��448LHClΪ20mol������Һ���Ϊ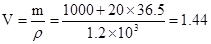 ��
�� ��
����2������C1V1=C2V2���ɵ�Ũ��Ϊ0.278 mol/L
��3����

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 L
L