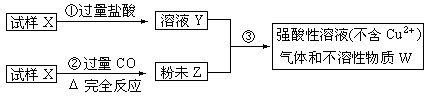
��Ŀ����
Ŀǰ������ʹ����㷺�Ľ���֮һ����֪�ڸ����£�Fe��ˮ�����ɷ�����Ӧ��
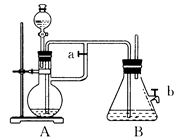
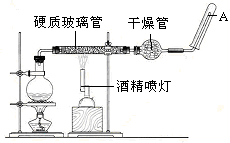
Ӧ������װ�ã���Ӳ�ʲ������з��뻹ԭ���ۺ�ʯ���Ļ������ȣ���ͨ��ˮ�������Ϳ�����ɸ����¡�Fe��ˮ�����ķ�Ӧʵ�顱��
��ش����е����⡣
�������ڱ��е�λ����_______
��д���÷�Ӧ�Ļ�ѧ����ʽ�� ��
��3���������������������ﷴӦԭ�������Ӹֹ죬�÷�Ӧ�Ļ�ѧ����ʽΪ
��4���������Ͳ���ɵĺϽ�a mol������Pt�����ʵ�������Ϊx���гɷ�ĩ״��ȫ��Ͷ�뺬bmol HNO3��ϡ��Һ�У�ʹ���ַ�Ӧ����HNO3�Ļ�ԭ����ֻ��NO���Իش��������⣺
����HNO3���������ӣ���Һ�еĽ������ӺͲ�������ijɷ�����������������������ӷ�����д�±��հף�
�ڵ�x="0.5" ������Һ��Fe3+��Fe2+�����ʵ�����ȣ��ڱ�״���¹�����112mLNO��
��a = ��b = ��
Ӧ������װ�ã���Ӳ�ʲ������з��뻹ԭ���ۺ�ʯ���Ļ������ȣ���ͨ��ˮ�������Ϳ�����ɸ����¡�Fe��ˮ�����ķ�Ӧʵ�顱��

��ش����е����⡣
�������ڱ��е�λ����_______
��д���÷�Ӧ�Ļ�ѧ����ʽ�� ��
��3���������������������ﷴӦԭ�������Ӹֹ죬�÷�Ӧ�Ļ�ѧ����ʽΪ
��4���������Ͳ���ɵĺϽ�a mol������Pt�����ʵ�������Ϊx���гɷ�ĩ״��ȫ��Ͷ�뺬bmol HNO3��ϡ��Һ�У�ʹ���ַ�Ӧ����HNO3�Ļ�ԭ����ֻ��NO���Իش��������⣺
����HNO3���������ӣ���Һ�еĽ������ӺͲ�������ijɷ�����������������������ӷ�����д�±��հף�
| | �� | �� | �� | �� |
| ��Һ�еĽ������� | | Fe2+ | | |
| ��������ɷ� | Fe��Pt | | Pt | Pt |
�ڵ�x="0.5" ������Һ��Fe3+��Fe2+�����ʵ�����ȣ��ڱ�״���¹�����112mLNO��
��a = ��b = ��
��1���������ڣ��ڢ� �� (1��)
��2��3Fe+4H2O(g) Fe3O4+4H2 (2��)
Fe3O4+4H2 (2��)
��3��2Al+Fe2O 3 Al2O3 + 2Fe (2��) (�𰸺���������)
Al2O3 + 2Fe (2��) (�𰸺���������)
��4����(2��)
��a =0.012��b = 0.02 (2��)
��2��3Fe+4H2O(g)
 Fe3O4+4H2 (2��)
Fe3O4+4H2 (2��)��3��2Al+Fe2O 3
 Al2O3 + 2Fe (2��) (�𰸺���������)
Al2O3 + 2Fe (2��) (�𰸺���������)��4����(2��)
| | �� | �� | �� | �� |
| ��Һ�еĽ������� | Fe2+ | Fe2+ | Fe2+ Fe3+ | Fe3+ |
| ��������ɷ� | Fe��Pt | Pt | Pt | Pt |
�����������1��Fe��Ԫ�����ڱ���λ�ڵ������ڣ��ڢ� �塣
��2��Fe��H2O��g����Ӧ����Fe3O4��H2����ѧ����ʽΪ��3Fe+4H2O(g)
 Fe3O4+4H2
Fe3O4+4H2��3���������������ﷴӦԭ�������Ӹֹ죬��ѧ����ʽΪ��2Al+Fe2O 3
 Al2O3 + 2Fe
Al2O3 + 2Fe ��4������Ϊ��Feʣ�࣬������Һ����Fe2+������HNO3�����࣬Feȫ����Ӧ������ֻ��Pt��������HNO3������Fe2+������ΪFe3+����Һ����Fe2+��Fe3+��HNO3�ﵽ������ȫ��ת��ΪFe3+��
����Һ��Fe3+��Fe2+�����ʵ�����ȣ���Fe2+�����ʵ���Ϊnmol����Fe3+ҲΪnmol������������ԭ��Ӧ�����غ��֪��2n+3n=0.112L��22.4L/mol��3����n=0.003mol��Fe2+��Fe3+��Ϊ0.003mol��Pt�����ʵ�������Ϊx=0.5����Pt�����ʵ���Ϊ0.006mol������a=0.003mol+0.003mol+0.006mol=0.012mol��HNO3��Ӧ��ת��ΪFe(NO3)2��Fe(NO3)3��NO������HNO3�����ʵ���b=0.003mol��2+0.003mol��3+0.112L��22.4L/mol=0.02mol��

��ϰ��ϵ�д�
 A�ӽ��� ϵ�д�
A�ӽ��� ϵ�д� ȫ�Ų��Ծ�ϵ�д�
ȫ�Ų��Ծ�ϵ�д�
�����Ŀ