��Ŀ����
A��B��C��D��E����Ԫ�����ڱ���ǰ20��Ԫ�أ���ԭ��������������B��C��Dͬ���ڣ�A��Dͬ���壬E������Ԫ�ؼȲ���ͬ����Ҳ����ͬ���壮B��C��D������������ˮ������ܻ������Ӧ�����κ�ˮ��������EA2��A-��ԭ�Ӻ���K���Ӳ��L���Ӳ��ϵĵ�����֮��Ϊ1��4��
��1��A��EԪ�ط��ŷֱ�Ϊ �� ��DԪ�ص�ԭ�ӽṹʾ��ͼ�� ��
��2��A��B��C��D����Ԫ�ص�ԭ�Ӱ뾶��С�����˳��Ϊ ����Ԫ�ط��ű�ʾ����
��3��A��D���⻯���У�ǰ�߷е�ϸߣ�ԭ���� ��
��4��C��D�γɵĻ�����ˮ��Һ�� �ԣ���ᡱ���������ԭ���ǣ������ӷ���ʽ��ʾ���� ��
��1��A��EԪ�ط��ŷֱ�Ϊ
��2��A��B��C��D����Ԫ�ص�ԭ�Ӱ뾶��С�����˳��Ϊ
��3��A��D���⻯���У�ǰ�߷е�ϸߣ�ԭ����
��4��C��D�γɵĻ�����ˮ��Һ��
������A��B��C��D��E����Ԫ�����ڱ���ǰ20��Ԫ�أ���ԭ��������������B��C��D������������ˮ������ܻ��෴Ӧ�����κ�ˮ��˵���������������������B��C��Dͬ���ڣ����ԭ���������ж�BΪNaԪ�أ�CΪAlԪ�أ�E������Ԫ�ؼȲ���ͬ����Ҳ����ͬ������ԭ���������ӦΪ��������Ԫ�أ�ǰ20��Ԫ����ֻ��Ca���ϣ���EΪCa��������EA2��A-��ԭ�Ӻ���K���Ӳ��L���Ӳ��ϵĵ�����֮��Ϊ1��4����A-�ĺ��������Ϊ2+2��4=10����AΪFԪ�أ�A��Dͬ���壬��DΪClԪ�أ��ݴ˽��
����⣺A��B��C��D��E����Ԫ�����ڱ���ǰ20��Ԫ�أ���ԭ��������������B��C��D������������ˮ������ܻ��෴Ӧ�����κ�ˮ��˵���������������������B��C��Dͬ���ڣ����ԭ���������ж�BΪNaԪ�أ�CΪAlԪ�أ�E������Ԫ�ؼȲ���ͬ����Ҳ����ͬ������ԭ���������ӦΪ��������Ԫ�أ�ǰ20��Ԫ����ֻ��Ca���ϣ���EΪCa��������EA2��A-��ԭ�Ӻ���K���Ӳ��L���Ӳ��ϵĵ�����֮��Ϊ1��4����A-�ĺ��������Ϊ2+2��4=10����AΪFԪ�أ�A��Dͬ���壬��DΪClԪ�أ�
��1��������������֪��A��EԪ�ط��ŷֱ�ΪF��Ca��DΪClԪ�أ���ԭ�ӽṹʾ��ͼ��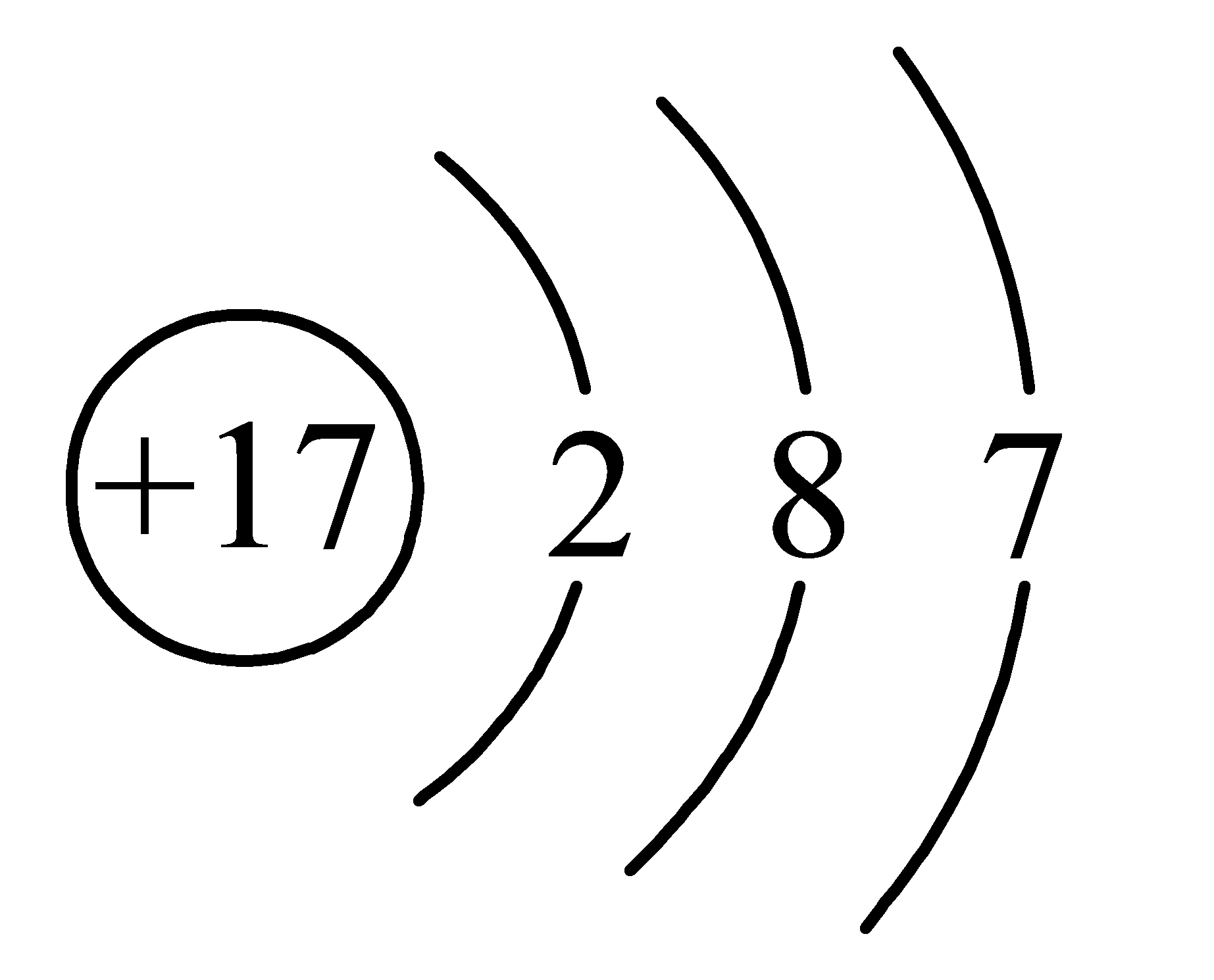 ��
��
�ʴ�Ϊ��F��Ca��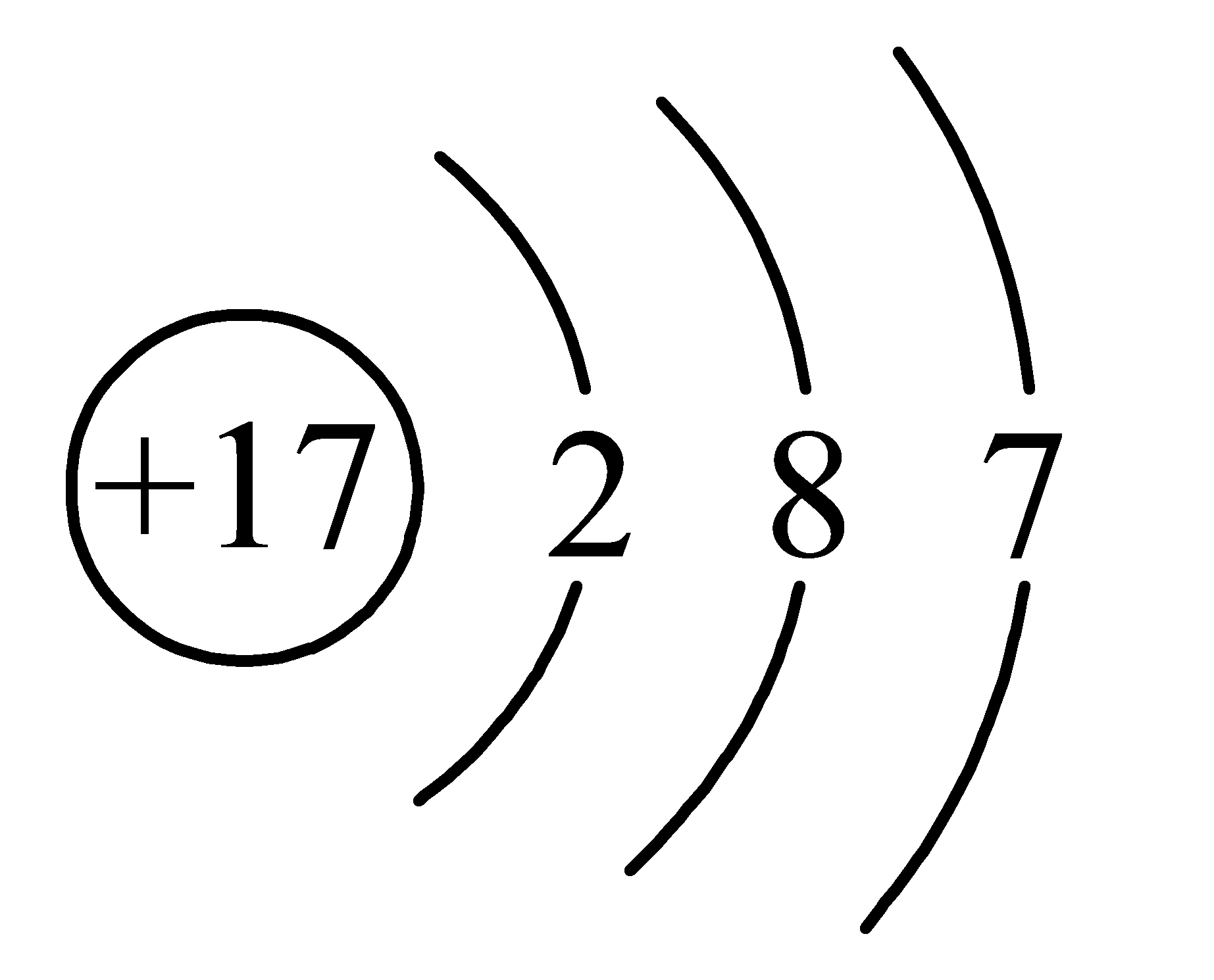 ��
��
��2��ͬ������ԭ����������ԭ�Ӱ뾶��С��ͬ�������϶���ԭ�Ӱ뾶����ԭ�Ӱ뾶F��Cl��Al��Na��
�ʴ�Ϊ��F��Cl��Al��Na��
��3��A��D���⻯��ֱ�ΪHF��HCl��HF����֮��������������ȷ��»�����ǿ����HF�ķе�ϸߣ�
�ʴ�Ϊ��HF����֮��������������ȷ��»�����ǿ��
��4��C��D�γɵĻ�����ΪAlCl3��ˮ��Һ��Al3+����ˮ�⣺Al3++3H2O?Al��OH��3+3H+���ƻ�ˮ�ĵ���ƽ�⣬��Һ�����ԣ�
�ʴ�Ϊ�����ԣ�Al3++3H2O?Al��OH��3+3H+��
��1��������������֪��A��EԪ�ط��ŷֱ�ΪF��Ca��DΪClԪ�أ���ԭ�ӽṹʾ��ͼ��
�ʴ�Ϊ��F��Ca��
��2��ͬ������ԭ����������ԭ�Ӱ뾶��С��ͬ�������϶���ԭ�Ӱ뾶����ԭ�Ӱ뾶F��Cl��Al��Na��
�ʴ�Ϊ��F��Cl��Al��Na��
��3��A��D���⻯��ֱ�ΪHF��HCl��HF����֮��������������ȷ��»�����ǿ����HF�ķе�ϸߣ�
�ʴ�Ϊ��HF����֮��������������ȷ��»�����ǿ��
��4��C��D�γɵĻ�����ΪAlCl3��ˮ��Һ��Al3+����ˮ�⣺Al3++3H2O?Al��OH��3+3H+���ƻ�ˮ�ĵ���ƽ�⣬��Һ�����ԣ�
�ʴ�Ϊ�����ԣ�Al3++3H2O?Al��OH��3+3H+��
���������⿼��ṹ����λ�ù�ϵӦ�ã��ѶȲ����ƶ�Ԫ���ǽ���ؼ������ضԻ���֪ʶ�Ĺ��̣�

��ϰ��ϵ�д�
 ����5��2���ϵ�д�
����5��2���ϵ�д�
�����Ŀ
 CH3COOH+OH-
CH3COOH+OH- ��֪A��B��C��D��E����Ԫ�����ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��Bԭ�ӵ�p�����������γɵ��⻯��ķе���ͬ����Ԫ�ص��⻯������͵ģ�Dԭ�ӵõ�һ�����Ӻ�3p���ȫ������A+��Dԭ���γɵ�������һ�����Ӳ㣮C��A�γ�A2C�����ӻ����E��ԭ������Ϊ26��Eԭ�ӻ�������Χ�н϶���������Ŀչ��������һЩ���ӻ������γ��������������������ش��������⣺������ʱ��A��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ��
��֪A��B��C��D��E����Ԫ�����ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��Bԭ�ӵ�p�����������γɵ��⻯��ķе���ͬ����Ԫ�ص��⻯������͵ģ�Dԭ�ӵõ�һ�����Ӻ�3p���ȫ������A+��Dԭ���γɵ�������һ�����Ӳ㣮C��A�γ�A2C�����ӻ����E��ԭ������Ϊ26��Eԭ�ӻ�������Χ�н϶���������Ŀչ��������һЩ���ӻ������γ��������������������ش��������⣺������ʱ��A��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ�� ʾ��λ�ڸ�������Ķ�������ģ��û�����Ļ�ѧʽ��
ʾ��λ�ڸ�������Ķ�������ģ��û�����Ļ�ѧʽ��
 ��֪A��B��C��D��E����Ԫ�����ڱ��е�ǰ������Ԫ�أ�����ԭ�������Ĵ�С��ϵΪA��C��B��D��E����֪Aԭ�ӵ�p���Ϊ����������γɵ��⻯��ķе���ͬ����ǽ���Ԫ�ص��⻯������ߵģ�Dԭ�ӵõ�һ�����Ӻ���3p�����ȫ������B+���ӱ�Dԭ���γɵ�������һ�����Ӳ㣮C��B���γ�BC�͵����ӻ����E��ԭ������Ϊ29��
��֪A��B��C��D��E����Ԫ�����ڱ��е�ǰ������Ԫ�أ�����ԭ�������Ĵ�С��ϵΪA��C��B��D��E����֪Aԭ�ӵ�p���Ϊ����������γɵ��⻯��ķе���ͬ����ǽ���Ԫ�ص��⻯������ߵģ�Dԭ�ӵõ�һ�����Ӻ���3p�����ȫ������B+���ӱ�Dԭ���γɵ�������һ�����Ӳ㣮C��B���γ�BC�͵����ӻ����E��ԭ������Ϊ29��