��Ŀ����
����Ŀ��������Դ�����þ��й���ǰ����
��1����ͼ�ǴӺ�ˮ����ȡþ�ļ����̣�
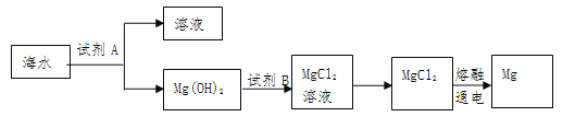
��Mg(OH)2ת��ΪMgCl2�����ӷ���ʽ��_______________��
�ڽ�MgCl2��Һ��________��������������ȴ���ᾧ�Ƶ�MgCl2���塣
������ˮMgCl2��ȡMg�Ļ�ѧ����ʽ��________________________��
��2���������и�����I����ʽ���ڵĵ�Ԫ�ء�ʵ������ȡI2��;��������ʾ��

�����պ������ҽ�ʱ���õ���Ҫ����������________________��
�����ữ����Һ�мӹ���������Һ��д���÷�Ӧ�����ӷ���ʽ��_____________________��
�۷�Ӧ�����ɼ���_________����ȡ����������ȡ����Һ�ķ����ӵ�ˮ����ȡ�⡣
���𰸡� Mg(OH)2 + 2H+=Mg2+ + 2H2O �Ȼ��� MgCl2 ![]() Mg + Cl2�� ���� 2H��+2I��+H2O2
Mg + Cl2�� ���� 2H��+2I��+H2O2![]() I2+2H2O CCl4��
I2+2H2O CCl4��
��������(1)��������þ��ϡ���ᷢ���кͷ�Ӧ�����Ȼ�þ��ˮ����Ӧ�����ӷ���ʽΪMg(OH)2 + 2H+=Mg2+ + 2H2O���ʴ�Ϊ��Mg(OH)2 + 2H+=Mg2+ + 2H2O��
���Ȼ�þˮ�����ɵ��Ȼ������ӷ���ֱ�Ӽ��Ȼ�ٽ�ˮ������������þ�����������Ҫ���Ȼ�����������������ȴ���ᾧ�Ƶ�MgCl2���壬�ʴ�Ϊ���Ȼ�����
����ˮMgCl2�����ȡMg��ͬʱ������������Ӧ�Ļ�ѧ����ʽΪ��MgCl2![]() Mg+Cl2�����ʴ�Ϊ�� MgCl2
Mg+Cl2�����ʴ�Ϊ�� MgCl2![]() Mg+Cl2����
Mg+Cl2����
(2)��ʵ������ȡI2��;����֪�����������������գ��ܽ����˵õ���I-����Һ���ӹ������������õ����ⵥ�ʵ���Һ��������ȡ�Ȳ����õ��⡣
�����պ������ҽ�ʱ���õ���Ҫ�����оƾ��ơ������������ǣ��ʴ�Ϊ����������
�����ữ����Һ�мӹ���������Һ����Ӧ�����ӷ���ʽΪH2O2+2H++2I-=I2+2H2O���ʴ�Ϊ��H2O2+2H++2I-=I2+2H2O��
�۷�Ӧ�����ɼ���CCl4���л��ܼ�����ȡ����������ȡ����Һ�ķ����ӵ�ˮ����ȡ�⣬�ʴ�Ϊ��CCl4����