��Ŀ����
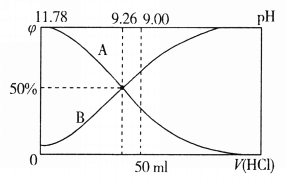
����Ŀ��������A���緢������ţ���У������������Ǵ�л���м��壬�����������������۵ȷ����Ƶá�A�ĸ���������ϲ���IJ��Ƽ�֮һ��A��ij�ִ����Ĵ����±�����������ﲻ�ܷ���������Ӧ����Ũ��������£� A�ɷ���������ʾ�ķ�Ӧ��
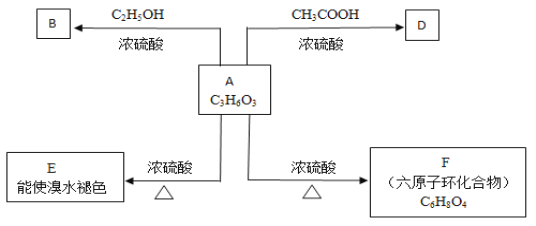
(1)A���еĹ���������Ϊ___________________,A��E�ķ�Ӧ����Ϊ _______��
(2)д��һ�ֺ�B������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽ________________��
(3)д��A��D�Ļ�ѧ����ʽ_____________________________________��
(4)F�Ľṹ��ʽΪ��_______________________________��
���𰸡��ǻ����Ȼ� ��ȥ��Ӧ HOCH2CH2COOCH2CH3 ��CH3CH2COOCH(OH)CH3��CH3CH2COOCH2CH2OH CH3CH(OH)COOH+CH3COOH![]() CH3CH(COOH)OOCCH3+H2O
CH3CH(COOH)OOCCH3+H2O 
��������
A��Ũ��������¼��ܺ��Ҵ���Ӧ�����ܺ����ᷴӦ��˵��A�м����Ȼ������ǻ���A�������IJ��ﲻ�ܷ���������Ӧ��˵���ǻ�����̼���Ķ˵��ϣ����ж�AΪ����CH3CH��OH��COOH����A�������ɵ�CH3COCOOH�����ܷ���������Ӧ����ͽ�һ��֤����A�����ᡣA���Ҵ�����������Ӧ����B��BΪCH3CH��OH��COOCH2CH3��A�����ᷢ��������Ӧ����D��DΪCH3COOCH��CH3��COOH��A��Ũ���ᡢ��������������E��E������ˮ��ɫ��Ӧ������ȥ��Ӧ��EΪCH2=CHCOOH��A��Ũ���ᡢ����������������ԭ�ӻ�״������F�����F�ķ���ʽ��֪��Ϊ2�������ᷢ��������Ӧ���ɻ�״�������FΪ ���ݴ˽��
���ݴ˽��
��1��������������֪��������AΪCH3CH��OH��COOH�����еĹ���������Ϊ�ǻ����Ȼ���A��E��������Ũ���ᡢ���������·�����Ӧ����CH2=CHCOOH��Ϊ��ȥ��Ӧ��
�ʴ�Ϊ���ǻ����Ȼ�����ȥ��Ӧ��
(2) BΪCH3CH��OH��COOCH2CH3����B������ͬ�����ŵ�ͬ���칹�壬�������ǻ����������ṹ��ʽ����ΪHOCH2CH2COOCH2CH3 ��CH3CH2COOCH(OH)CH3��CH3CH2COOCH2CH2OH��
�ʴ�Ϊ��HOCH2CH2COOCH2CH3 ��CH3CH2COOCH(OH)CH3��CH3CH2COOCH2CH2OH��
(3 A�����ᷢ��������Ӧ����D����ѧ����ʽΪCH3CH(OH)COOH+CH3COOH![]() CH3CH(COOH)OOCCH3+H2O��
CH3CH(COOH)OOCCH3+H2O��
�ʴ�Ϊ��CH3CH(OH)COOH+CH3COOH![]() CH3CH(COOH)OOCCH3+H2O��
CH3CH(COOH)OOCCH3+H2O��
(4)A��F��������Ũ���ᡢ���������·���������Ӧ���ɻ�״������F����F�Ľṹ��ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��