��Ŀ����
��֪�������£�A�����ҺpH=a��B�����ҺpH=b��
(1)��AΪ���ᣬBΪ������������a=3��b=11�����ߵ������ϣ���Һ��pHΪ ��
a.����7 b.����7 c.��7
(2)��AΪ���ᣬBΪ�������ƣ���a=4��b=12����ôA��Һ��ˮ�������������Ũ��Ϊ mol/L,B��Һ��ˮ�������������Ũ��Ϊ mol/L��
(3)��A�Ļ�ѧʽΪHR��B�Ļ�ѧʽΪMOH����a+b=14�����ߵ������Ϻ���Һ�Լ��ԡ�������Һ�бض���һ�������ܷ���ˮ�⣬��ˮ�ⷴӦ�����ӷ���ʽΪ ��
��1)b (2)10-10 10-12 (3)M++H2O MOH+H+
MOH+H+
����

 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д���ҵ̼����(����ԼΪ98%)�к���Ca2����Mg2����Fe3����Cl����SO42�������ʣ��ᴿ�����������£�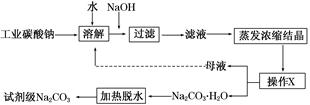
��.̼���Ƶı�����Һ�ڲ�ͬ�¶�����������������ͼ��ʾ��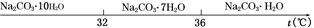
��.�й����ʵ��ܶȻ����£�
| ���� | CaCO3 | MgCO3 | Ca(OH)2 | Mg(OH)2 | Fe(OH)3 |
| Ksp | 4.96��10��9 | 6.82��10��6 | 4.68��10��6 | 5.61��10��12 | 2.64��10��39 |
�ش��������⣺
(1)����NaOH��Һ����˵õ�����������Ҫ����________(��д��ѧʽ)��25��ʱ������Mg2����Fe3������Һ�еμ�NaOH��Һ�������ֳ�����������Һ��pH��8 ʱ��c(Mg2��)��c(Fe3��)��________��
(2)����XΪ________�����¶�Ӧ������_____________________________________
(3)���˴ӡ���ɫ��ѧ���Ƕ����뽫��ĸҺ����������������ʾ����ѭ��ʹ�á��������ʵ�ʹ�ҵ�������Ƿ����________����˵������______________________________
________________________________________________________________________��
��ͼ��ʾ������ƿ�зֱ�װ�뺬��̪��0.01 mol��L-1 CH3COONa��Һ,���ֱ������ʢ��ˮ���ձ���,Ȼ�����ձ����м�����ʯ��,���ձ����м���NH4NO3����,�ձ����в����κ����ʡ�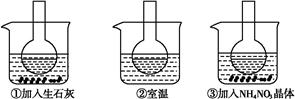
(1)����̪��0.01 mol��L-1 CH3COONa��Һ��dz��ɫ��ԭ��Ϊ
(2)ʵ������з�����ƿ������Һ��ɫ����,��ƿ������Һ��ɫ��dz,������������ȷ��������������
| A��ˮ�ⷴӦΪ���ȷ�Ӧ | B��ˮ�ⷴӦΪ���ȷ�Ӧ |
| C��NH4NO3����ˮʱ�ų����� | D��NH4NO3����ˮʱ�������� |
�±��Ǽ���������ʵĵ���ƽ�ⳣ�������ܵ���ʵ��ܶȻ�Ksp��25�棩��
| ����� | ���뷽��ʽ | ���볣��K | Ksp |
| H2CO3 | H2CO3 HCO3����H�� HCO3����H��HCO3��  CO32����H�� CO32����H�� | K1��4.31��10��7 K2��5.61��10��11 | �� |
| C6H5OH | C6H5OH C6H5O����H�� C6H5O����H�� | 1.1��10��10 | �� |
| H3PO4 | H3PO4 H2PO4����H�� H2PO4����H��H2PO4��  HPO42����H�� HPO42����H��HPO42��  PO43����H�� PO43����H�� | K1��7.52��10��3 K2��6.23��10��6 K1��2.20��10��13 | �� |
| NH3��H2O | NH3��H2O OH����NH4�� OH����NH4�� | 1.76��10��5 | �� |
| BaSO4 | BaSO4��s�� Ba2����SO42�� Ba2����SO42�� | �� | 1.07��10��10 |
��1��д��C6H5OH��Na3PO4��Ӧ�����ӷ���ʽ��_________________��
��2��25��ʱ����10 mL 0. 01 mol/LC6H5OH��Һ�еμ�V mL 0.1 mol/L��ˮ�������Һ������Ũ�ȹ�ϵ��ȷ����__________������ţ���
a�������ҺpH��7,��V��10
b��V��5ʱ��2c��NH3��H2O����2c��NH4������c��C6H5OH����c��C6H5O����
c��V��10ʱ�����Һ��ˮ�ĵ���̶�С��0.01 mol
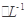 C6H5OH��Һ��ˮ�ĵ���̶�
C6H5OH��Һ��ˮ�ĵ���̶�d�������ҺpH��7����c��NH4������c��C6H5O������c��H������c��OH����
��3��ˮ�ⷴӦ�Ļ�ѧƽ�ⳣ����Ϊˮ�ⳣ������Kb��ʾ������Ȼ�ѧƽ�ⳣ���Ķ��塣25��ʱ��Na2CO3��һ��ˮ�ⷴӦ��ˮ�ⳣ��Kb��____mol/L��
��4����ͼ��ʾ����T1��T2��ͬ�¶�������BaSO4��ˮ�еij����ܽ�ƽ�����ߣ���֪BaSO4��Ksp���¶����߶�����
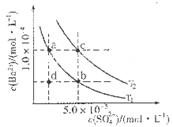
��T2____ 25�棨���������������������
������T1�¶�ʱBaSO4�ij����ܽ�ƽ�����ߣ�����˵����ȷ����____������ţ���
a������Na2SO4����ʹ��Һ��a���Ϊb��
b����T1�����Ϸ����������ߣ�����һ��ʱ������BaSO4��������
c�������ܼ�����ʹ��Һ��d���Ϊ������a��b֮���ijһ�㣨����a��b��
d�����¿�ʹ��Һ��b���Ϊd��


 H����B2�����ش��������⡣
H����B2�����ش��������⡣