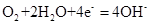��Ŀ����
��14�֣�ij�о�С�������������о���
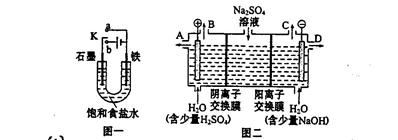
��1�������ϳ�ʱ���ͬѧ�۲쵽�������ǣ���ͼ�е������������������ ������ĸ����
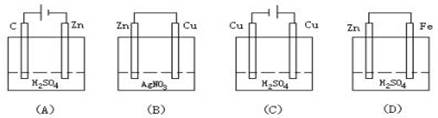
��2������ʵ�������жϣ�����������ʴ�����У������ĵ缫��ӦΪ�� ��
��3����Ϊ�˷�ֹ�������⣬��С��ͬѧ���������������һ��������ý��������
A. �� B. ͭ C. п
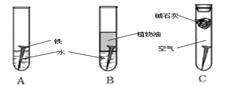
��4����������ʴ����ҵ�����г���Ը������С�����������������Ч�������������ĸ�ʴ����ν���������������ڸ�������Ƚ�������������ʹ������γ�һ�����ܵ�����ɫ����Ĥ�������������̿ɱ�ʾ���£�
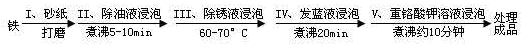
Ϊ���龭������������������Ƿ�ϸ�����Ʒ�������5%������ͭ��Һ�������Ʒ���ϸ�����������С�ɿף�δ�γ����ܵ�����Ĥ����һ��ʱ�佫�۲쵽������Ϊ__________________________��

��1�������ϳ�ʱ���ͬѧ�۲쵽�������ǣ���ͼ�е������������������ ������ĸ����
��2������ʵ�������жϣ�����������ʴ�����У������ĵ缫��ӦΪ�� ��
��3����Ϊ�˷�ֹ�������⣬��С��ͬѧ���������������һ��������ý��������
A. �� B. ͭ C. п
��4����������ʴ����ҵ�����г���Ը������С�����������������Ч�������������ĸ�ʴ����ν���������������ڸ�������Ƚ�������������ʹ������γ�һ�����ܵ�����ɫ����Ĥ�������������̿ɱ�ʾ���£�
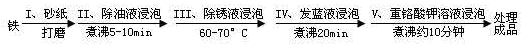
Ϊ���龭������������������Ƿ�ϸ�����Ʒ�������5%������ͭ��Һ�������Ʒ���ϸ�����������С�ɿף�δ�γ����ܵ�����Ĥ����һ��ʱ�佫�۲쵽������Ϊ__________________________��
��1��A ��3�֣� ��2��O2��2 H2O��4e���� 4 OH����3�֣�
��3��C ��3�֣� ��4������Ʒ�����к�ɫ����������3�֣�
��3��C ��3�֣� ��4������Ʒ�����к�ɫ����������3�֣�
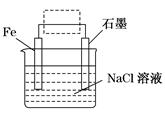
��1��ֲ��������ֹ��������ʯ��������CO2��ˮ��ʵ������������ʴ����A��
��2��ˮ�����Եģ���������������������ʴ��������ӦʽΪO2��2 H2O��4e���� 4 OH����
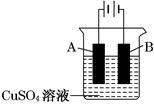
��3���Ʋ�������ʽ������ǿ�ڵ�����������ʹ�Ʋ����𣬷����绯ѧ��ʴ����Ҳ������������������˴�ѡC��
��4��������ϸ��������ܺ�����ͭ�����û���Ӧ�����ɺ�ɫ��ͭ���������������Ʒ�����к�ɫ����������
��2��ˮ�����Եģ���������������������ʴ��������ӦʽΪO2��2 H2O��4e���� 4 OH����
��3���Ʋ�������ʽ������ǿ�ڵ�����������ʹ�Ʋ����𣬷����绯ѧ��ʴ����Ҳ������������������˴�ѡC��
��4��������ϸ��������ܺ�����ͭ�����û���Ӧ�����ɺ�ɫ��ͭ���������������Ʒ�����к�ɫ����������

��ϰ��ϵ�д�
�����Ŀ
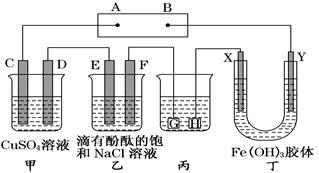
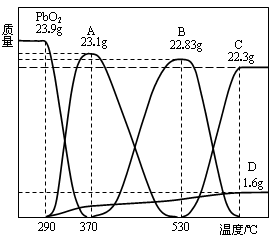
 2Ni(OH)2 �����ݴ˷�Ӧ�жϣ����������в���ȷ���ǣ� ��
2Ni(OH)2 �����ݴ˷�Ӧ�жϣ����������в���ȷ���ǣ� ��