��Ŀ����
��18mol/LŨ��������100ml3.0mol/Lϡ�����ʵ�������²��裺
�ټ�������Ũ����������ϡ�͢���ȡһ�������Ũ����ܶ��ݡ�ҡ�Ȣ�ת�ơ�ϴ��
�ش���������
��1�������������ȷ˳����______��
��2����ʵ���õ��IJ���������______��
��3����ʵ������Ũ����������______ml��
��ȡŨ�������õ���Ͳ�Ĺ����______��������ű�ʾ������������ѡ��A.10mlB.25mlC.50mlD.100ml��
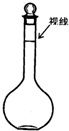
��4��ijͬѧ�۲�Һ��������ͼ��ʾ����������ҺŨ���к�Ӱ�죿���ƫ�ߡ�����ƫ�͡�������Ӱ�족��______��
��û�н��в�������ݣ���______���ƫ�ߡ�����ƫ�͡�������Ӱ�족����
��5����������ˮʱ���������˿̶��ߣ�Ӧ��β�����______��
�ټ�������Ũ����������ϡ�͢���ȡһ�������Ũ����ܶ��ݡ�ҡ�Ȣ�ת�ơ�ϴ��
�ش���������
��1�������������ȷ˳����______��
��2����ʵ���õ��IJ���������______��
��3����ʵ������Ũ����������______ml��
��ȡŨ�������õ���Ͳ�Ĺ����______��������ű�ʾ������������ѡ��A.10mlB.25mlC.50mlD.100ml��
��4��ijͬѧ�۲�Һ��������ͼ��ʾ����������ҺŨ���к�Ӱ�죿���ƫ�ߡ�����ƫ�͡�������Ӱ�족��______��
��û�н��в�������ݣ���______���ƫ�ߡ�����ƫ�͡�������Ӱ�족����
��5����������ˮʱ���������˿̶��ߣ�Ӧ��β�����______��

��1�����ݼ��㡢��ȡ��ϡ�͡���Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ��������������������˳��Ϊ���٢ۢڢݢܣ�
�ʴ�Ϊ���٢ۢڢݢܣ�
��2����100mL����ƿ������Һ������Ͳ��ȡŨ���ᣬ���ձ�ϡ��Ũ���ᣬ�ò�����������������ý�ͷ�ιܶ��ݣ�
�ʴ�Ϊ��100mL����ƿ����Ͳ���ձ�������������ͷ�ιܣ�
��3����Ũ��������ΪV��18mol/L��V=3.0mol/L��0.1L��V=0.0167L=16.7mL��ѡȡ����Ͳ���Ӧ�õ��ڻ�������Ũ��������������ѡȡ25mL��Ͳ���ʴ�Ϊ��16.7��B��
��4��ijͬѧ�۲�Һ��������ͼ��ʾ����Һ�����ƫС��������ҺŨ��ƫ�ߣ�
��û�н��в�������ݣ����ʵ����ʵ���ƫС��������ҺŨ��ƫ�ͣ�
�ʴ�Ϊ��ƫ�ߣ�ƫ�ͣ�
��5����������ˮʱ���������˿̶��ߣ�Ӧ�������ƣ��ʴ�Ϊ���������ƣ�
�ʴ�Ϊ���٢ۢڢݢܣ�
��2����100mL����ƿ������Һ������Ͳ��ȡŨ���ᣬ���ձ�ϡ��Ũ���ᣬ�ò�����������������ý�ͷ�ιܶ��ݣ�
�ʴ�Ϊ��100mL����ƿ����Ͳ���ձ�������������ͷ�ιܣ�
��3����Ũ��������ΪV��18mol/L��V=3.0mol/L��0.1L��V=0.0167L=16.7mL��ѡȡ����Ͳ���Ӧ�õ��ڻ�������Ũ��������������ѡȡ25mL��Ͳ���ʴ�Ϊ��16.7��B��
��4��ijͬѧ�۲�Һ��������ͼ��ʾ����Һ�����ƫС��������ҺŨ��ƫ�ߣ�
��û�н��в�������ݣ����ʵ����ʵ���ƫС��������ҺŨ��ƫ�ͣ�
�ʴ�Ϊ��ƫ�ߣ�ƫ�ͣ�
��5����������ˮʱ���������˿̶��ߣ�Ӧ�������ƣ��ʴ�Ϊ���������ƣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ