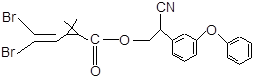��Ŀ����
��9�֣���1������֧���Ļ�����A�ķ���ʽΪC4H6O2��A����ʹBr2�����Ȼ�̼��Һ��ɫ��1molA��1molNaHCO3����ȫ��Ӧ����A�Ľṹ��ʽ��___________��д����A������ͬ�����ŵ�A������ͬ���칹��Ľṹ��ʽ____________________��
��2��������B��C��H��O����Ԫ�أ�������Ϊ60������̼����������Ϊ60%�������������Ϊ13.3%��B�ڴ���Cu�������±�����������C��C�ܷ���������Ӧ����B�Ľṹ��ʽ��_________��д��C����������Ӧ�Ļ�ѧ����ʽ��_______________________________��
��3��D��NaOHˮ��Һ�м��ȷ�Ӧ��������A�����κ�B����Ӧ��Ӧ�Ļ�ѧ����ʽ��___________________________��
��2��������B��C��H��O����Ԫ�أ�������Ϊ60������̼����������Ϊ60%�������������Ϊ13.3%��B�ڴ���Cu�������±�����������C��C�ܷ���������Ӧ����B�Ľṹ��ʽ��_________��д��C����������Ӧ�Ļ�ѧ����ʽ��_______________________________��
��3��D��NaOHˮ��Һ�м��ȷ�Ӧ��������A�����κ�B����Ӧ��Ӧ�Ļ�ѧ����ʽ��___________________________��
��9�֣���1��CH2=C(CH3)COOH �� CH2=CHCH2COOH��CH3CH="CHCOOH"
��2��CH3CH2CH2OH
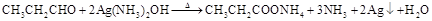
��3��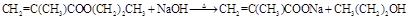
��2��CH3CH2CH2OH
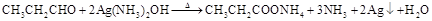
��3��
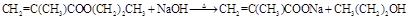
��1���ӷ���ʽ�Ͽɿ��������Ͷ�Ϊ2��1molA��1mol NaHCO3����ȫ��Ӧ��˵�������к���һ���Ȼ���A����ʹBr2�����Ȼ�̼��Һ��ɫ��˵��������һ��̼̼˫��������A����֧��������ΪCH2=C(CH3)COOH
��2�������е�̼ԭ�Ӹ�����60��60%/12=3����ԭ�Ӹ�����60��13.3%/12=8����ԭ��Ϊ����60-36-8��/16=1�����Է���ʽΪ��C3H8O����B�ڴ���Cu�������±�����������C��C�ܷ���������Ӧ��˵��B�к��С�CH2OH���ţ���BΪ��CH3CH2CH2OH
��2�������е�̼ԭ�Ӹ�����60��60%/12=3����ԭ�Ӹ�����60��13.3%/12=8����ԭ��Ϊ����60-36-8��/16=1�����Է���ʽΪ��C3H8O����B�ڴ���Cu�������±�����������C��C�ܷ���������Ӧ��˵��B�к��С�CH2OH���ţ���BΪ��CH3CH2CH2OH

��ϰ��ϵ�д�
 һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
�����Ŀ


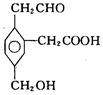 ����һ�������¿��ܷ����ķ�Ӧ�У�
����һ�������¿��ܷ����ķ�Ӧ�У�