��Ŀ����
��ѧ�ҷ���ijҩ��M��������Ѫ�ܼ�������Ϊ�������������ͷų�һ�֡���ʹ���ӡ�D����������D�������ڵ�����ԭ����Ϊ�������ٻ���1998��ŵ��������ѧ��ҽѧ����������������⣺
(1)��֪M����Է�������Ϊ227����C��H��O��N����Ԫ����ɣ�C��H��N��������������Ϊ15.86%��2.20%��18.50%����M�ķ���ʽ��__________________________��D��˫ԭ�ӷ��ӣ���Է�������Ϊ30����D�ķ���ʽΪ_______________________________��
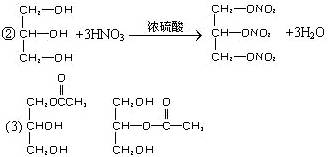
(2)��֬A��ͼ2-3��ʾ;���ɵõ�M��

ͼ2-3
ͼ�Тڵ���ʾ��
C2H5OH+HO��NO2![]() C2H5O��NO2+H2O
C2H5O��NO2+H2O
���� ��������
��Ӧ�ٵĻ�ѧ����ʽ��_____________________________________________________��
��Ӧ�ڵĻ�ѧ����ʽ��_____________________________________________________��
(3)C��B��������һ�������·�Ӧ���ɵĻ������Է�������Ϊ134��д��C���п��ܵĽṹ��ʽ��___________________________________________________________
(4)����0.1 mol B�������Ľ����Ʒ�Ӧ����������___________________g�����ơ�
˼·���������M��C��H��O��N����Ԫ����ɣ������������ͷų�һ�֡���ʹ���ӡ�D��D��˫ԭ�ӷ��ӡ���Է�������Ϊ30����Щ��Ϣ��֪��D����Ԫ��һ����M������Ԫ�ص����֣��������������ֻ��NO�������⡣
��֬ˮ��ɵ�֬������ͣ�����Bһ���Ǹ��ͣ������ʾ���ݺ�M����Է������������ж�MΪ�����������������ͬ����˼·���ɵó���3���Ĵ𰸡�
�𰸣�(1)C3H5O9N3 NO
��2����

��4��6.9





