��Ŀ����
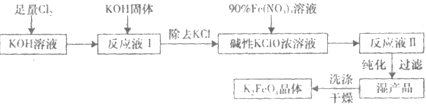
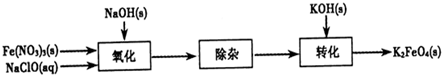
������أ�K2FeO4����һ�ּ�������������������ɱ���������ȥ�ǡ���ɫ������Ϊһ������͡���Ч����ɫ�����Ķ��ˮ����������ʮ���������ҹ��Ը������������ˮ�����е�Ӧ�õ��о�Ҳ�������룬��ȡ�ÿ�ϲ�ɹ����Ƚ�������Ʊ������Ǵ�������������������KOH��Һ��ͨ������Cl2�Ʊ�������ر�����Һ���ٷִμ���KOH���壬�õ��������ǿ���Ա�����Һ�������������Σ��ϳɸ�����أ�
��1����������ǿ�����Һ�м����������η�����Ӧ�����ӷ���ʽ��
��Fe3++3OH-=Fe��OH������______��
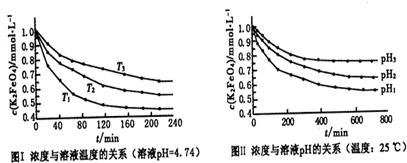
��2�������������ˮ���ͷŴ�����ԭ�������Ӷ��dz���Ч��ɱ��ˮ�еIJ����Ͳ��������ͬʱ����������ԭ������̬��Fe��OH��3������һ��Ʒ�������������������ܸ�Ч�س�ȥˮ�е�ϸ�����������K2Fe2O4��Һ��pH=4.74����Һ�У����Ƴ�c��FeO2-4��=1.0mmol?L-1�������������ֱ�����20�桢30�桢40���60��ĺ���ˮԡ�У��ⶨc��FeO+2-4���ı仯�������ͼ�����������ˮ��Ӧ�����ӷ�Ӧ����ʽΪ______���÷�Ӧ�ġ�H______0���������������=������
��3���������λ���һ��������ܵ�صIJ��ϣ��������ɵĵ�������ߣ��ŵ�������ܳ�ʱ�䱣���ȶ��ķŵ��ѹ��������ص��ܷ�ӦΪ��3Zn+2K2FeO4+8H2O
3Zn��OH��3+2Fe��OH��3+4KOH�õ�طŵ�ʱ�ĸ�����ӦʽΪ______�������·��5.418��1022������ͨ������������______g������ز��뷴Ӧ��
��4���ⶨijK2FeO4��ҺŨ�ȵ�ʵ�鲽�����£�
����1��ȷ��ȡV mL K2FeO4��Һ���뵽��ƿ��
����2����ǿ������Һ�У��ù���CrO-2��FeO2-4��Ӧ����Fe��OH��3��CrO2-4
����3��������ϡ���ᣬʹCrO2-4ת��ΪCr2O2-7��CrO-2ת��ΪCr3+��Fe��OH��3ת��ΪFe2+
����4�������������������ָʾ������c mol?L-1��NH4��2Fe��SO4��2����Һ�ζ����յ㣬���ģ�NH4��2Fe��SO4��2��ҺV1mL��
�ٵζ�ʱ������Ӧ�����ӷ���ʽΪ______��
��ԭ��Һ��K2FeO4��Ũ��Ϊ______���ú���ĸ�Ĵ���ʽ��ʾ����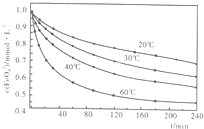
��1����������ǿ�����Һ�м����������η�����Ӧ�����ӷ���ʽ��
��Fe3++3OH-=Fe��OH������______��
��2�������������ˮ���ͷŴ�����ԭ�������Ӷ��dz���Ч��ɱ��ˮ�еIJ����Ͳ��������ͬʱ����������ԭ������̬��Fe��OH��3������һ��Ʒ�������������������ܸ�Ч�س�ȥˮ�е�ϸ�����������K2Fe2O4��Һ��pH=4.74����Һ�У����Ƴ�c��FeO2-4��=1.0mmol?L-1�������������ֱ�����20�桢30�桢40���60��ĺ���ˮԡ�У��ⶨc��FeO+2-4���ı仯�������ͼ�����������ˮ��Ӧ�����ӷ�Ӧ����ʽΪ______���÷�Ӧ�ġ�H______0���������������=������
��3���������λ���һ��������ܵ�صIJ��ϣ��������ɵĵ�������ߣ��ŵ�������ܳ�ʱ�䱣���ȶ��ķŵ��ѹ��������ص��ܷ�ӦΪ��3Zn+2K2FeO4+8H2O
| �ŵ� |
| ��� |
��4���ⶨijK2FeO4��ҺŨ�ȵ�ʵ�鲽�����£�
����1��ȷ��ȡV mL K2FeO4��Һ���뵽��ƿ��
����2����ǿ������Һ�У��ù���CrO-2��FeO2-4��Ӧ����Fe��OH��3��CrO2-4
����3��������ϡ���ᣬʹCrO2-4ת��ΪCr2O2-7��CrO-2ת��ΪCr3+��Fe��OH��3ת��ΪFe2+
����4�������������������ָʾ������c mol?L-1��NH4��2Fe��SO4��2����Һ�ζ����յ㣬���ģ�NH4��2Fe��SO4��2��ҺV1mL��
�ٵζ�ʱ������Ӧ�����ӷ���ʽΪ______��
��ԭ��Һ��K2FeO4��Ũ��Ϊ______���ú���ĸ�Ĵ���ʽ��ʾ����

��1��������ؾ���ǿ�������ԣ�����������֮��ķ�ӦΪ��2Fe��OH��3+3ClO-+10OH-=2FeO2-4+3Cl-+5H2O���ʴ�Ϊ��2Fe��OH��3+3ClO-+10OH-=2FeO2-4+3Cl-+5H2O��
��2�������������ˮ���ͷŴ�����ԭ�������������������ʣ����ͬʱ����������ԭ������̬��Fe��OH��3����ӦΪ��4FeO42-+10H2O=4Fe��OH��3+8OH-+3O2��������ͼ����Կ��������ŷ�Ӧ�Ľ��У���Ӧ��ϵ���¶����ͣ����Է�Ӧ�����ȷ�Ӧ���ʴ�Ϊ��4FeO42-+10H2O=4Fe��OH��3+8OH-+3O2��������
��3���õ�طŵ�ʱ����ԭ��صĹ���ԭ������ԭ����У���������ʧ���ӵ�������Ӧ����Zn+2OH--2e-=Zn��OH���������Ϸ�����ӦΪ��2K2FeO4+8H2O+6e-��2Fe��OH��3+6OH-������������Ӧ�������·��5.418��1022����0.09mol����ͨ��ʱ�����뷴Ӧ�ĸ�����ص�����Ϊ��
g=5.94g���ʴ�Ϊ��Zn+2OH--2e-=Zn��OH����5.94��
��4���ٵζ�ʱ��Cr2O72-���к�ǿ�������ԣ��ܽ�������������Ϊ���������ӣ�������Ӧ�����ӷ���ʽΪ��6Fe2++Cr2O2-7+14H+=6Fe3++2Cr3++7H2O��
�ʴ�Ϊ��6Fe2++Cr2O2-7+14H+=6Fe3++2Cr3++7H2O��
����ǿ������Һ�У��ù���CrO-2��FeO2-4��Ӧ����Fe��OH��3��CrO42-��CrO2-+FeO42-+2H2O=Fe��OH��3��+CrO42-+OH-���ζ�ʱ��������Ӧ�����ӷ���ʽΪ��6Fe2++Cr2O2-7+14H+=6Fe3++2Cr3++7H2O������K2FeO4��Һ�ͣ�NH4��2Fe��SO4��2����Һ���Ĺ�ϵ���ɵó�ԭ��Һ��K2FeO4��Ũ��Ϊ
mol/L���ʴ�Ϊ��
mol/L��
��2�������������ˮ���ͷŴ�����ԭ�������������������ʣ����ͬʱ����������ԭ������̬��Fe��OH��3����ӦΪ��4FeO42-+10H2O=4Fe��OH��3+8OH-+3O2��������ͼ����Կ��������ŷ�Ӧ�Ľ��У���Ӧ��ϵ���¶����ͣ����Է�Ӧ�����ȷ�Ӧ���ʴ�Ϊ��4FeO42-+10H2O=4Fe��OH��3+8OH-+3O2��������
��3���õ�طŵ�ʱ����ԭ��صĹ���ԭ������ԭ����У���������ʧ���ӵ�������Ӧ����Zn+2OH--2e-=Zn��OH���������Ϸ�����ӦΪ��2K2FeO4+8H2O+6e-��2Fe��OH��3+6OH-������������Ӧ�������·��5.418��1022����0.09mol����ͨ��ʱ�����뷴Ӧ�ĸ�����ص�����Ϊ��
| 2��0.09��198 |
| 6 |
��4���ٵζ�ʱ��Cr2O72-���к�ǿ�������ԣ��ܽ�������������Ϊ���������ӣ�������Ӧ�����ӷ���ʽΪ��6Fe2++Cr2O2-7+14H+=6Fe3++2Cr3++7H2O��
�ʴ�Ϊ��6Fe2++Cr2O2-7+14H+=6Fe3++2Cr3++7H2O��
����ǿ������Һ�У��ù���CrO-2��FeO2-4��Ӧ����Fe��OH��3��CrO42-��CrO2-+FeO42-+2H2O=Fe��OH��3��+CrO42-+OH-���ζ�ʱ��������Ӧ�����ӷ���ʽΪ��6Fe2++Cr2O2-7+14H+=6Fe3++2Cr3++7H2O������K2FeO4��Һ�ͣ�NH4��2Fe��SO4��2����Һ���Ĺ�ϵ���ɵó�ԭ��Һ��K2FeO4��Ũ��Ϊ
| cV1 |
| 3V |
| cV1 |
| 3V |

��ϰ��ϵ�д�
�����Ŀ