��Ŀ����
��13�֣���.�����и������ʣ�
E�������Ǻ��� F����CH3��2CH2��C��CH3��4
G�����ۺ���ά�� H��Һ��������
��1�� �������ʻ�Ϊͬλ�� ��2�� �������ʻ�Ϊͬ��������
��3�� ������������ͬϵ�� ��4�� ���������ʻ�Ϊͬ���칹��
��5�� ���е�������ͬһ���ʣ�6��D������һ����������Ӧ��һ����ѧ����ʽ
����ͼ��ʾ4��̼ԭ�����ϵķ�ʽ��С���ʾ̼ԭ�ӣ�С����ʾ��ѧ��������̼ԭ��������Ļ�ѧ�����������ϡ�

��1��ͼ�������������� ��������ͬ������ϩ������ ��
��2����ͼ����B��Ϊͬ���칹�嵫������ͬ��������ʵ��ǣ� ��
��ij�л������庬̼82.7%������17.3�����ڱ�״���������ܶ���2.59g/L����1�����л������Է�������Ϊ__________;(2)���л���ķ���ʽΪ____________������ܵĽṹ��ʽΪ ��
| A��O2��O3�������� |
B�� |
| C��CH3CH2CH2CH3��CH3CH(CH3)2 |
D�� |
G�����ۺ���ά�� H��Һ��������
��1�� �������ʻ�Ϊͬλ�� ��2�� �������ʻ�Ϊͬ��������
��3�� ������������ͬϵ�� ��4�� ���������ʻ�Ϊͬ���칹��
��5�� ���е�������ͬһ���ʣ�6��D������һ����������Ӧ��һ����ѧ����ʽ
����ͼ��ʾ4��̼ԭ�����ϵķ�ʽ��С���ʾ̼ԭ�ӣ�С����ʾ��ѧ��������̼ԭ��������Ļ�ѧ�����������ϡ�

��1��ͼ�������������� ��������ͬ������ϩ������ ��
��2����ͼ����B��Ϊͬ���칹�嵫������ͬ��������ʵ��ǣ� ��
��ij�л������庬̼82.7%������17.3�����ڱ�״���������ܶ���2.59g/L����1�����л������Է�������Ϊ__________;(2)���л���ķ���ʽΪ____________������ܵĽṹ��ʽΪ ��
�� B �� A�� F��CE��DH �� CH2Br2����l����CHBr2Cl+HCl
�� AC��BEF��H
��58 C4H10 CH3(CH2)2CH3 CH3CH(CH3)CH3
�� AC��BEF��H
��58 C4H10 CH3(CH2)2CH3 CH3CH(CH3)CH3
����������ͬ����������ͬ��ͬһ��Ԫ�صIJ�ͬ���ػ�Ϊͬλ�أ�����B��ȷ����ͬһ��Ԫ���γɵIJ�ͬ���ʣ�����Ϊͬ�������壬����A��ȷ������ʽ��ͬ���ṹ��ͬ�Ļ����ﻥΪͬ���칹�壬���CE��ȷ���ṹ���ƣ��������������ɸ�CH2ԭ���ŵ�ͬһ�����ʻ�Ϊͬϵ�����F��ȷ�����ʺͽṹ����ȫ��ͬ������ͬһ�����ʣ����DH��ȷ��D�к�����ԭ�ӣ��ɷ���ȡ����Ӧ������ʽΪCH2Br2����l����CHBr2Cl+HCl��
��1���������̼ԭ��֮���Ե�����ϳ���״��ֱ����֧����������ϼ�ȫ��Ϊ��ԭ�������ͣ���������������������AC��ȷ������1��̼̼˫����������ϩ��������BEF��ȷ��
��2��B�Ƕ�ϩ���ַ���ʽΪC4H8��������B��Ϊͬ���칹�嵫������ͬ��������ʵ���H���������顣
���ڱ�״���������ܶ���2.59g/L������Է���������2.59��22.4��58������̼ԭ�Ӻ���ԭ�ӵĸ����ֱ�Ϊ ��
��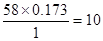 ����˷���ʽΪC4H10�����ܵĽṹ��ʽΪCH3(CH2)2CH3��CH3CH(CH3)CH3��
����˷���ʽΪC4H10�����ܵĽṹ��ʽΪCH3(CH2)2CH3��CH3CH(CH3)CH3��
��1���������̼ԭ��֮���Ե�����ϳ���״��ֱ����֧����������ϼ�ȫ��Ϊ��ԭ�������ͣ���������������������AC��ȷ������1��̼̼˫����������ϩ��������BEF��ȷ��
��2��B�Ƕ�ϩ���ַ���ʽΪC4H8��������B��Ϊͬ���칹�嵫������ͬ��������ʵ���H���������顣
���ڱ�״���������ܶ���2.59g/L������Է���������2.59��22.4��58������̼ԭ�Ӻ���ԭ�ӵĸ����ֱ�Ϊ
 ��
��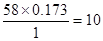 ����˷���ʽΪC4H10�����ܵĽṹ��ʽΪCH3(CH2)2CH3��CH3CH(CH3)CH3��
����˷���ʽΪC4H10�����ܵĽṹ��ʽΪCH3(CH2)2CH3��CH3CH(CH3)CH3��
��ϰ��ϵ�д�
 99��1������ĩ��ѵ��ϵ�д�
99��1������ĩ��ѵ��ϵ�д� ��ǿ��У��ĩ���100��ϵ�д�
��ǿ��У��ĩ���100��ϵ�д� �óɼ�1��1��ĩ���100��ϵ�д�
�óɼ�1��1��ĩ���100��ϵ�д� ��״Ԫ���źþ�ϵ�д�
��״Ԫ���źþ�ϵ�д�
�����Ŀ
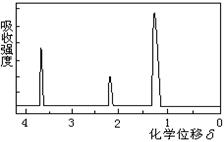
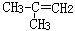




 ��
��