��Ŀ����
�����£�Ksp��Mg��OH��2��=1.2×10-11mol3?L-3 Ksp��AgCl��=1.8×10-10mol2?L-2Ksp��Ag2CrO4��=1.9×10-12mol3?L-3��Ksp��CH3COOAg��=2.3×10-3mol2?L-2����������ȷ���ǣ� ��
A����������Ũ�Ⱦ�Ϊ0.2 mol?L-1��AgNO3��Һ��CH3COONa��Һһ������CH3COOAg����
B����0.001 mol?L-1��AgNO3��Һ����0.001 mol?L-1��KCl��0.001 mol?L-1��K2CrO4��Һ���Ȳ���Ag2CrO4����
C����Mg2+Ϊ0.121 mol?L-1����Һ��Ҫ����Mg��OH��2��������Һ��pH����Ҫ������9����
D����AgClˮ��Һ�м���NaCl��Һ��Ksp��AgCl�����
���𰸡�������A������Ksp��CH3COOAg��=c��CH3COO-��×c��Ag+�����㣻
B������Ksp��AgCl���Լ�Ksp��Ag2CrO4���������ӵ�Ũ����֮�������ܶȻ�ʱ�����ɳ�����
C������Ksp��Mg��OH��2��=c��Mg2+��×c2��OH-�����㣻
D��Ksp��AgCl��ֻ���¶ȵ�Ӱ�죮
����⣺A����������Ũ�Ⱦ�Ϊ0.1mol?L-1��c��CH3COO-��×c��Ag+��=0.01��2.3×10-3��һ������CH3COOAg��������A��ȷ��
B����0.001 mol?L-1��AgNO3��Һ����0.001 mol?L-1��KCl��0.001 mol?L-1��K2CrO4��Һ�У�c��Ag+��×c��Cl-��=10-6��c2��Ag+��×c��CrO42-��=10-9��AgCl�ȴﵽ���ͣ�Ӧ������AgCl��������B����
C��Ksp��Mg��OH��2��=c��Mg2+��×c2��OH-����c��OH-����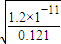 =10-5 mol?L-1����Һ��pH����Ҫ������9���ϣ���C����
=10-5 mol?L-1����Һ��pH����Ҫ������9���ϣ���C����
D��Ksp��AgCl��ֻ���¶ȵ�Ӱ�죬��Ũ���أ���D����
��ѡA��
�����������ۺϿ������ܵ���ʵ��ܽ�ƽ�⣬�����ڼ��㣬��Ŀ�Ѷ��еȣ�ע����ռ��㹫ʽ��Ӧ�ã�
B������Ksp��AgCl���Լ�Ksp��Ag2CrO4���������ӵ�Ũ����֮�������ܶȻ�ʱ�����ɳ�����
C������Ksp��Mg��OH��2��=c��Mg2+��×c2��OH-�����㣻
D��Ksp��AgCl��ֻ���¶ȵ�Ӱ�죮
����⣺A����������Ũ�Ⱦ�Ϊ0.1mol?L-1��c��CH3COO-��×c��Ag+��=0.01��2.3×10-3��һ������CH3COOAg��������A��ȷ��
B����0.001 mol?L-1��AgNO3��Һ����0.001 mol?L-1��KCl��0.001 mol?L-1��K2CrO4��Һ�У�c��Ag+��×c��Cl-��=10-6��c2��Ag+��×c��CrO42-��=10-9��AgCl�ȴﵽ���ͣ�Ӧ������AgCl��������B����
C��Ksp��Mg��OH��2��=c��Mg2+��×c2��OH-����c��OH-����
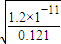 =10-5 mol?L-1����Һ��pH����Ҫ������9���ϣ���C����
=10-5 mol?L-1����Һ��pH����Ҫ������9���ϣ���C����D��Ksp��AgCl��ֻ���¶ȵ�Ӱ�죬��Ũ���أ���D����
��ѡA��
�����������ۺϿ������ܵ���ʵ��ܽ�ƽ�⣬�����ڼ��㣬��Ŀ�Ѷ��еȣ�ע����ռ��㹫ʽ��Ӧ�ã�

��ϰ��ϵ�д�
�����Ŀ