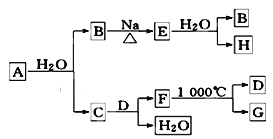
��Ŀ����
����Ŀ�������к��зḻ�ĵ⡣ij�о���ѧϰС�鰴��ͼ��ʾʵ��������ȡ�����еĵ⡣

�ش��������⣺
��1����������պ���ʱ�������ż��⣬����Ҫ�õ����������е�________������ĸ��ţ���
A���ձ� B������ C�������� D�������� E���ƾ��� F��������
��2������۵�ʵ�����������________��
��3������ܷ�Ӧ�����ӷ���ʽΪ____________________________��
��4��������У������ñ�����ȡ���������__________________����ˮ��Һ���뱽������ֹ�ɹ۲쵽��ʵ������Ϊ________��
��5�������ʵ�������ȡ����ˮ��Һ���Ƿ��е��ʵ�__________________��
���𰸡� BDE ���� 2I����MnO2��4H����Mn2����I2��2H2O ����ˮ�������ܣ����ڱ��е��ܽ�ȱ���ˮ�еĴ� �ֲ㣬�ϲ�Ϊ��ɫ���²�ӽ���ɫ ȡ������ȡ����ˮ��Һ���Թ��У����뼸�ε�����Һ�������Һ������˵�����е��ʵ⣻�����Һ��������˵��û�е��ʵ�
����������1����ͼ �����պ�����װ��ͼ�����������������ϣ������������ż��ϣ��·��ռ�žƾ��Ƽ��ȣ���ѡBDE��
�����պ�����װ��ͼ�����������������ϣ������������ż��ϣ��·��ռ�žƾ��Ƽ��ȣ���ѡBDE��
��2��������Ƿ�������Һ��õ��������ӵ���Һ��ʵ����������ǹ��ˡ�
��3�������Ӿ��л�ԭ�ԣ�MnO2���н�ǿ�������ԣ������������·���������ԭ��Ӧ��������ת���ɵⵥ���������غ㷨����д�����ӷ���ʽ��2I����MnO2��4H����Mn2����I2��2H2O��
��4������ˮ���������ҵ��ڱ��е��ܽ�ȱ���ˮ�д���������ͨ����ȡ���ñ�����ȡ�⣻�ⵥ�����ڱ�����Һ��ɫ����ұ����ܶȱ�ˮ���ܶ�С����˵�ˮ��Һ���뱽������ֹ�ɹ۲쵽��ʵ������Ϊ��Һ��ֲ㣬�ϲ�Ϊ��ɫ���²�ӽ���ɫ��
��5��������Һ��I2����ɫ�����õ�����Һ������ⵥ����ȡ������ȡ����ˮ��Һ���Թ��У����뼸�ε�����Һ�������Һ������˵�����е��ʵ⣻�����Һ��������˵��û�е��ʵ���