��Ŀ����
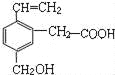
����Ŀ����1��ijϩ�������������ӳɷ�Ӧ�ɵõ��ṹ��ʽΪ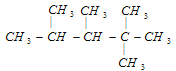
�����������ϩ�����ܵĽṹ��ʽ�ǣ�___________��___________��___________��
��2��ij��A������ͼ��������Է�������Ϊ84��������ױ��������к���̼̼˫�����˴Ź����ױ���������ֻ��һ�����͵��⡣
����ϵͳ��������A������ ��
��A�е�̼ԭ���Ƿ���ͬһƽ�棿 ����ǡ����ߡ����ǡ�����
��3����A��B��C��D��E 5��������ȡ0.01 mol���ȼ�պ�B��C��E�������Ķ�����̼��Ϊ448 mL����״������A��Dȼ�����õĶ�����̼����ǰ�ߵ�3�������������������£�A��B��C���ܺ����������ӳɷ�Ӧ������A ����ת��ΪD��B����ת��ΪC��E��C����ת��ΪE��B��C����ʹ�������������Һ��ɫ����A��D��E�����ʣ�����м����ʱA���巢��ȡ����Ӧ��
��д�����Ľṹ��ʽ��B��___________��C��___________��D��___________�� E��___________��
��д��A���巴Ӧ����ʽ______________________________
���𰸡���1��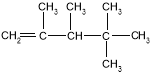
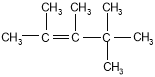
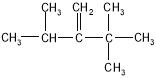
��2����2,3-����-2-��ϡ �� ��
��3����CH��CH CH2=CH2 ![]() CH3CH3
CH3CH3
��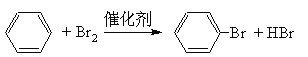
��������
�����������1��ijϩ�����������ӳɷ�Ӧ�ɵõ��ṹ��ʽΪ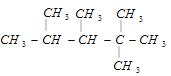 ���������ڼ���Ĺ���������̼�Ǽܲ������仯��ȷ����Ӧϩ���ķ���Ϊ����ȥ���γ�̼̼˫����ע������ͬ�ṹ��������˫��ʱ̼ԭ�Ӳ��ܳ���4�����ۼ������ϩ�����ܵĽṹ��ʽ��
���������ڼ���Ĺ���������̼�Ǽܲ������仯��ȷ����Ӧϩ���ķ���Ϊ����ȥ���γ�̼̼˫����ע������ͬ�ṹ��������˫��ʱ̼ԭ�Ӳ��ܳ���4�����ۼ������ϩ�����ܵĽṹ��ʽ��


 ��
��
��2��ij��A������ͼ��������Է�������Ϊ84���������෨ȷ�������ķ���ʽΪC6H12��������ױ��������к���̼̼˫��������ϩ�����˴Ź����ױ���������ֻ��һ�����͵�������ϩ���Ľṹ��ʽΪ(CH3)2C=C(CH3)2������ϵͳ��������A����Ϊ2,3-����-2-��ϩ����������ϩ���ӵ�ƽ���νṹ����A�е�̼ԭ�Ӷ�����ͬһƽ�档
��3�����������Ϣ�ƶ�ȡ0.01 mol�����ȼ�պ�B��C��E�������Ķ�����̼��Ϊ448 mL����״���������ʵ���Ϊ0.02mol����B��C��E�����к���2��̼ԭ�ӣ�B��C���ܺ����������ӳɷ�Ӧ��B����ת��ΪC��E��C����ת��ΪE����BΪ��Ȳ��CΪ��ϩ��EΪ������A��Dȼ�����õĶ�����̼����ǰ�ߵ�3������A��D�����к���6��̼ԭ�ӣ�����м����ʱA���巢��ȡ����Ӧ����AΪ����A�ܺ����������ӳɷ�Ӧת��ΪD��DΪ������������Ӧ���Ľṹ��ʽ��B��CH��CH�� C��CH2=CH2��D��![]() �� E��CH3CH3��
�� E��CH3CH3��
������Һ�����������������·���ȡ����Ӧ�����屽���廯�⣬��ѧ����ʽΪ
![]() ��
��