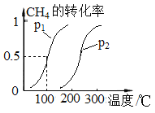
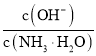��Ŀ����
����Ŀ������������������۷���Ϣ�صijɷ�֮һ�������㽶����ζ��ʵ�����Ʊ������������ķ�Ӧ��
![]() +
+![]()
![]()
![]() +H2O
+H2O
װ��ʾ��ͼΪ��
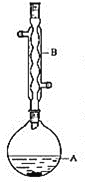
�й��������£�
��Է������� | �ܶ�/(gcm-3) | �е�/�� | ˮ���ܽ��� | |
���촼 | 88 | 0.8123 | 131 | �� |
���� | 60 | 1.0492 | 118 | �� |
���������� | 130 | 0.8670 | 142 | ���� |
ʵ�鲽�裺
��A�м���![]() ���촼
���촼![]() ������Ũ�����
������Ũ�����![]() Ƭ���Ƭ����ʼ��������A������50min����ӦҺ�������º����Һ©���У��ֱ�������ˮ������̼��������Һ��ˮϴ�ӣ��ֳ��IJ������������ˮ
Ƭ���Ƭ����ʼ��������A������50min����ӦҺ�������º����Һ©���У��ֱ�������ˮ������̼��������Һ��ˮϴ�ӣ��ֳ��IJ������������ˮ![]() ���壬����Ƭ�̣����˳�ȥ
���壬����Ƭ�̣����˳�ȥ![]() ���壬�����������ռ�
���壬�����������ռ�![]() ��֣�����������֬
��֣�����������֬![]()
�ش��������⣺
(1)����B��������_________
(2)��ϴ�Ӳ����У���һ��ˮϴ����ҪĿ����_____���ڶ���ˮϴ����ҪĿ����_____
(3)��ϴ�ӡ���Һ�����У�Ӧ�����Ȼ���ã����ֲ��(����)_______
a��ֱ�ӽ���������֬�ӷ�Һ©�����Ͽڵ���
b��ֱ�ӽ���������ӷ�Һ�˶����¿ڷų�
c���Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ���������֬���¿ڷų�
d���Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ���������Ӵ��Ͽڵ���
(4)��ʵ���м�����������Ŀ����_____________
(5)ʵ���м���������ˮ![]() ��Ŀ����____________
��Ŀ����____________
(6)����������У�����ѡ��װ����ȷ����_________![]() ����
����![]() ��
��
a. 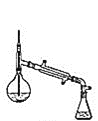 b.
b. 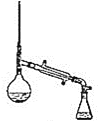 c.
c. 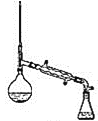 d.
d. 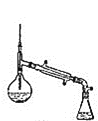
(7)��ʵ��IJ�����________![]() ����
����![]()
![]() .
.![]() .
.![]()
(8)�ڽ����������ʱ������![]() �㿪ʼ�ռ���֣���ʹʵ��IJ���ƫ_________
�㿪ʼ�ռ���֣���ʹʵ��IJ���ƫ_________![]() ����������������
����������������![]() ����ԭ����___________
����ԭ����___________
���𰸡����������� ϴ��������ʹ��� ϴ��̼������ d ��ߴ���ת���� ���� b c �� ���ռ�������δ��Ӧ�����촼
��������
(1)��װ��ʾ��ͼ��֪װ��B�����������������ܣ��ݴ˽��
(2)��Ӧ�����ҺҪ�������ϴ�ӣ���ϴ�Ӳ����У���һ��ˮϴ����ҪĿ���dz�ȥ�ִ��������Ϊ��Ӧ�Ĵ��ᣬ�ñ���̼��������Һ�ȿ��Գ�ȥδϴ���Ĵ��ᣬҲ���Խ��������ܽ�ȣ��ٵڶ���ˮϴ����ҪĿ���dz�ȥ��Ʒ�ϲ�����̼�����ƣ��ݴ˽��
(3)���������ܶȱ�ˮС�����������ܣ����ˮ���²㣬�����ϲ㡣��Һʱ��Ҫ�Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ��������������Ͽڷų����ݴ˽��
(4)������Ӧ������������Ŀ������ߴ���ת���ʣ��ݴ˽��
(5)ʵ���м���������ˮ����þ��Ŀ��������������������ˮ�֣�������и�����ݴ˽��(6)����������У��¶ȼƵ�ˮ����Ҫ��������ƿ��֧�ܿڸ������ų�a��d������c��ʹ�õ������������ܣ�����ʹ��Ʒ����������ȫ���ռ�����ƿ�У��ݴ˽��
(7) ![]() ��
�� ![]() �����ڶ��߷�Ӧʱ��
�����ڶ��߷�Ӧʱ��![]() ��Ӧ�ģ��������������Ҫ���մ������㣬�ݴ˽��
��Ӧ�ģ��������������Ҫ���մ������㣬�ݴ˽��
(8)�ڽ����������ʱ������![]() ��ʼ�ռ���֣���ʱ�������к��д������ռ�������δ��Ӧ�����촼����˲���ƫ�ߣ��ݴ˽��
��ʼ�ռ���֣���ʱ�������к��д������ռ�������δ��Ӧ�����촼����˲���ƫ�ߣ��ݴ˽��
(1)��װ��ʾ��ͼ��֪װ��B�����������������ܣ��ʴ�Ϊ�����������ܣ�
(2)��Ӧ�����ҺҪ�������ϴ�ӣ���ϴ�Ӳ����У���һ��ˮϴ����ҪĿ���dz�ȥ�ִ��������Ϊ��Ӧ�Ĵ��ᣬ�ñ���̼��������Һ�ȿ��Գ�ȥδϴ���Ĵ��ᣬҲ���Խ��������ܽ�ȣ��ٵڶ���ˮϴ����ҪĿ���dz�ȥ��Ʒ�ϲ�����̼�����ƣ��ʴ�Ϊ��ϴ��������ʹ��ϴ��̼�����ƣ�
(3)���������ܶȱ�ˮС�����������ܣ����ˮ���²㣬�����ϲ㡣��Һʱ��Ҫ�Ƚ�ˮ��ӷ�Һ©�����¿ڷų�����������Һ�����ʱ�رշ�Һ©���Ļ������ٽ��������������Ͽڷų���ѡ��D�������⣬�ʴ�Ϊ��D��
(4)������Ӧ�ǿ��淴Ӧ������Ӧ���Ũ�ȿ���ʹƽ�������ƶ���������һ�ַ�Ӧ���Ũ�ȣ���ʹ��һ�ַ�Ӧ���ת������ߣ���˱�ʵ���м�����������Ŀ������ߴ���ת���ʣ��ʴ�Ϊ����ߴ���ת���ʣ�
(5)ʵ���м���������ˮ����þ��Ŀ��������������������ˮ�֣�������и���ʴ�Ϊ�����
(6)����������У��¶ȼƵ�ˮ����Ҫ��������ƿ��֧�ܿڸ������ų�a��d������c��ʹ�õ������������ܣ�����ʹ��Ʒ����������ȫ���ռ�����ƿ�У��������ѡ��װ����ȷ����b���ʴ�Ϊ��b��
(7) ![]() ��
�� ![]() �����ڶ��߷�Ӧʱ��
�����ڶ��߷�Ӧʱ��![]() ��Ӧ�ģ��������������Ҫ���մ������㣬
��Ӧ�ģ��������������Ҫ���մ������㣬 ![]() �����Ա�ʵ��IJ�����
�����Ա�ʵ��IJ�����![]() ��ѡ��c�������⣬�ʴ�Ϊ��c��
��ѡ��c�������⣬�ʴ�Ϊ��c��
(8)�ڽ����������ʱ������![]() ��ʼ�ռ���֣���ʱ�������к��д������ռ�������δ��Ӧ�����촼����˲���ƫ�ߣ��ʴ�Ϊ���ߣ����ռ�������δ��Ӧ�����촼��
��ʼ�ռ���֣���ʱ�������к��д������ռ�������δ��Ӧ�����촼����˲���ƫ�ߣ��ʴ�Ϊ���ߣ����ռ�������δ��Ӧ�����촼��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ������������ѧ��Ӧ��ƽ�ⳣ����K1��K2��K3�����¶ȵĹ�ϵ�ֱ����±���ʾ��
��ѧ��Ӧ | ƽ�ⳣ�� | �¶� | |
973K | 1173K | ||
��Fe��s��+CO2��g�� | K1 | 1.47 | 2.15 |
��Fe��s��+H2O��g�� | K2 | 2.38 | 1.67 |
��CO��g��+H2O��g�� | K3 | �� | �� |
������˵����ȷ����
A����H1��0����H2��0
B����Ӧ�٢ڢ��ķ�Ӧ�������ϵ����H2����H1����H3
C����Ӧ�٢ڢ���ƽ�ⳣ�������ϵ��K1��K2��K3
D��Ҫʹ��Ӧ����һ�������½�����ƽ��������Ӧ�����ƶ����ɲ�ȡ���´�ʩ