��Ŀ����
����Ŀ������ͼ��ʾװ�ý���ʵ�飬����ȡ����������
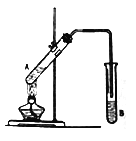
��1���Թ�A�е�Һ���������Լ���϶���:��2mL�Ҵ�����3mLŨ�����2mL ���ᡣһ����£��������Լ��ļ���˳���ǣ��ȼ���________������ţ���ͬ�����ټ���_________��������ۡ�
��2��Ϊ��ֹ�Թ�A�е�Һ����ʵ��ʱ�������У��ڼ���ǰ��Ӧ�������Ƭ�������Ⱥ���δ�������Ƭ��Ӧ��ȡ�IJ��ȴ�ʩ�ǣ�_________________________________________________________��
��3���Թ�B��ʢ�ŵ��Լ���___________________����Ӧ��������B�е�Һ��������Ҫ�õ��IJ���������Ҫ��_____________���Թ�B�еĵ���ĩ�˲�����Һ���·���Ŀ����_______________��
��4���Թ� A��CH3COOH��C2H518OH��Ӧ�Ļ�ѧ����ʽΪ:___________________________________��
��5����ʵ������30gCH3COOH��46gC2H5OH��Ӧ�����ʵ�ʵõ�������������������26.4g����ʵ�������������IJ�����________��
���𰸡� �� �� ֹͣ���ȣ���װ����ȴ�������Ƭ�������¼��� ����Na2CO3��Һ ��Һ©�� ��ֹ���� CH3CH218OH+CH3COOH ![]() CH3CO18OC2H5+H2O 60%
CH3CO18OC2H5+H2O 60%
����������1����ȡ����������Ϊ��ֹŨ���ᱻϡ�ͷų������ȵ���Һ�ηɽ���Ӧ�ȼ����Ҵ��ټ���Ũ���ᡣ
��2��Ϊ��ֹ�Թ�A�е�Һ����ʵ��ʱ�������У��ڼ���ǰ�ɼ������Ƭ�������Ⱥ���δ�������Ƭ���ɲ��ӣ������ǣ�ֹͣ���ȣ���װ����ȴ�������Ƭ�������¼�����
��3���Թ�B��ʢ�ű���Na2CO3��Һ���շ�Ӧ����������������ͬʱ��ȥ�ӷ������Ҵ������ᣬ��������������ˮ��Һ�����÷�Һ©�����룻Ϊ�˷�ֹ�������Թ�B�еĵ���ĩ�˲�������Һ��������
��4������������Ӧԭ��������ʧ�ǻ���ʧ�⡱�ɵ�CH3COOH��C2H518OH��Ӧ�Ļ�ѧ����ʽΪ��CH3CH218OH+CH3COOH ![]() CH3CO18OC2H5+H2O��
CH3CO18OC2H5+H2O��
��5��30gCH3COOH��0.5mol����46gC2H5OH��1mol����Ӧ��������������������44g��0.5mol����ʵ�ʵõ�������������������26.4g���ʸ�ʵ�������������IJ�������26.4g��44g��100%=60%��

����Ŀ����ͼ����ʾ��װ�ý���ʵ��,ʵ��������Ԥ����һ������( )

ѡ�� | ���е����� | ���е����� | Ԥ������ |
A | Ũ��ˮ | FeCl3��Һ | �����к��ɫ���� |
B | Ũ��ˮ | Ũ���� | �����а��� |
C | Ũ���� | ����KI��Һ | ������Һ�����Ա仯 |
D | Ũ���� | ��̪��Һ | ������Һ�����Ա仯 |
A. A B. B C. C D. D




