��Ŀ����
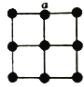
����Ŀ��H3AsO4ˮ��Һ�к���ĸ����ֵķֲ�����(ƽ��ʱij���ֵ�Ũ��ռ������Ũ��֮�͵ķ���)��pH�Ĺ�ϵ����ͼ��ʾ(��֪pKa����lgKa)������˵����ȷ����

A. H3AsO4��ҺpKa2Ϊ4.5
B. NaH2AsO4��Һ�Լ���
C. �����£�m���Ӧ��Һ����ˮ�������OH��Ũ��Ϊ10��11.5 mol/L
D. n���Ӧ��Һ�У�����Ũ�ȹ�ϵ��c(HAsO42��)��c(H2AsO4��)>c( OH��) ��c(H+)
���𰸡�D
��������
A�����ͼ��n�㣬H3AsO4��Һ��![]() ����pKa2Ϊ7����A����
����pKa2Ϊ7����A����
B����ͼ��֪pHΪ4ʱ����ҺΪNaH2AsO4��Һ��Ӧ�����ԣ���B����
C��m���Ӧ��ҺΪHAsO42-��AsO43-�Ļ����Һ����Һ�Լ��ԣ�����ˮ��ٽ�ˮ�ĵ��룬m����Һ��pH=11.5�����Ӧ��Һ����ˮ�������c(OH-)Ϊ10-2.5 mol��L- 1����C����
D��n��ʱ��ͼ���ϵ����ҺpH=7��֪c(HAsO42��)��c(H2AsO4��)>c(OH��)��c(H+)����D��ȷ��
����ѡD��

����Ŀ���Ƶij������ϼ�Ϊ��3�ۣ��ҹ��̲��ŷḻ�ĺ��ƿ�ʯ(Y2FeBe2Si2O10)����ҵ��ͨ�������������̿ɻ�������ơ�

��֪���ٸ��������йؽ��������γ������������ʱ��pH���±���
���� | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
Fe3�� | 2.1 | 3.1 |
Y3�� | 6.0 | 8.2 |
����Ԫ�����ڱ��У���Ԫ�غ���Ԫ�ش��ڵڶ����ں͵������ڵĶԽ���λ�ã���ѧ�������ơ�
(1)д��Na2SiO3��һ����;________________________��
(2)����Na2SiO3��Na2BeO2�����Һ���Ƶ�Be(OH)2������
�� ���ѡ�������_______�����Լ�����ͨ����Ҫ�IJ�������ʵ�֡�
A��NaOH��Һ B����ˮ C��CO2 D��HNO3
�� д��Na2BeO2���������ᷢ����Ӧ�����ӷ���ʽ___________________________��
(3)�����£���ӦFe3����3H2O(g) ![]() Fe (OH)3����3H+��ƽ�ⳣ��K= ______��ΪʹFe3��������ȫ���ð�ˮ����pH��aʱ��aӦ������_________��Χ�ڣ������Ӱ�ˮ����pH =b������Ӧ�����ӷ���ʽΪ____________________________��
Fe (OH)3����3H+��ƽ�ⳣ��K= ______��ΪʹFe3��������ȫ���ð�ˮ����pH��aʱ��aӦ������_________��Χ�ڣ������Ӱ�ˮ����pH =b������Ӧ�����ӷ���ʽΪ____________________________��
(4)���ղ�����ʱ�����ֽⷴӦ����������Ϊ�����ƣ����������ʹ����ʯ��ˮ����ǡ�д��������[Y2(C2O4)3��nH2O]���յĻ�ѧ����ʽ___________________________________��
����Ŀ����A��B��C��D��E��F����Ԫ�أ����ǵ������Ϣ���±���
Ԫ�ش��� | �����Ϣ |
A | �����ĵ������Ǵ�����������3�� |
B | ��ˮ�к�����һλ�Ľ���Ԫ�� |
C | L���1�����Ӻ��Ϊ�ȶ��ṹ |
D | ���������Ӻ�����18������ |
E | ʧȥһ�����Ӻ�ͳ�Ϊһ������ |
F | ����Ϊ����ɫ���壬���д̼�����ζ |
����д���пո�
��1��Aԭ�ӵĵ���ʽ��_____��
��2��B���ӵĽṹʾ��ͼ��_______��
��3��CԪ�ص����ƣ�___��Cԭ����������ߵĵ���λ�ڵ�______�㣬��C���������������������ͬ����������______���������ű�ʾ����
��4��D�Ķ��������ӵĵ���ʽ��___��DԪ�ص�ij��ͬλ��ԭ��������Ϊ34����ԭ�Ӻ��ڵ�������Ϊ_____��
��5��A��B��E����Ԫ���γɵĻ����ﳣ��������F���ʣ���Ӧ�Ļ�ѧ����ʽ��_____________��