��Ŀ����
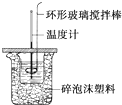
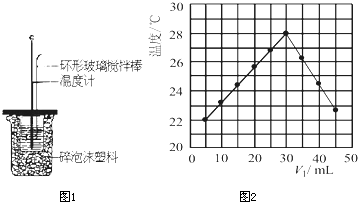
ijʵ��С����0.50mol/LNaOH��Һ��0.50mol/L������Һ�ⶨ�к��ȣ��ⶨ�к��ȵ�ʵ��װ����ͼ1��ʾ��
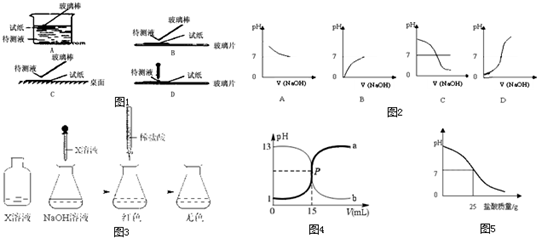
��1��д��ϡ�����ϡ����������Һ��Ӧ��ʾ�к��ȵ��Ȼ�ѧ����ʽ���к�����ֵΪ57.3kJ/mol����______��
��2��ȡ50mLNaOH��Һ��30mL������Һ����ʵ�飬�������±���ʾ��
������д�±��еĿհף�
�ڽ�����Ϊ0.50mol/LNaOH��Һ��0.50mol/L������Һ���ܶȶ���1g/cm3���кͺ�������Һ�ı�����c=4.18J/��g?�棩��������1molH2O�ġ�H=______kJ/mol��ȡС�����һλ����
������ʵ��������ֵ��57.3kJ/mol��ƫ�����ƫ���ԭ�������______������ĸ����
�����µ���10��ʱ����ʵ�飬������ȡNaOH��Һ�����ʱ���Ӷ������۷ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У���ʵ��ʱ�û���ͭ˿��������滷�β����������
��3����V1mL 0.4mol/LH2SO4��Һ��V2mLδ֪Ũ�ȵ�NaOH��Һ��Ͼ��Ⱥ��������¼��Һ�¶ȣ�ʵ������ͼ2��ʾ��ʵ����ʼ�ձ���V1+V2=50mL��������������ȷ����______
A����ʵ��ʱ�����¶�Ϊ22��
B��ʵ�������ѧ�ܿ�ת��Ϊ����
CNaOH��Һ��Ũ��ԼΪ1.2mol/L
D��ʵ�������ˮ���ɵķ�Ӧ���Ƿ��ȷ�Ӧ��

��1��д��ϡ�����ϡ����������Һ��Ӧ��ʾ�к��ȵ��Ȼ�ѧ����ʽ���к�����ֵΪ57.3kJ/mol����______��
��2��ȡ50mLNaOH��Һ��30mL������Һ����ʵ�飬�������±���ʾ��
������д�±��еĿհף�
| �¶� | ��ʼ�¶�t1/�� | ��ֹ�¶� t1/�� | �¶Ȳ� ��t2-t1��t1/�� | ||
| ʵ����� | H2SO4 | NaOH | ƽ��ֵ | ||
| 1 | 26.2 | 26.0 | 26.1 | 30.1 | ______ |
| 2 | 27.0 | 27.4 | 27.2 | 33.3 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.8 | |
| 4 | 26.4 | 26.2 | 26.3 | 30.4 | |
������ʵ��������ֵ��57.3kJ/mol��ƫ�����ƫ���ԭ�������______������ĸ����
�����µ���10��ʱ����ʵ�飬������ȡNaOH��Һ�����ʱ���Ӷ������۷ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У���ʵ��ʱ�û���ͭ˿��������滷�β����������
��3����V1mL 0.4mol/LH2SO4��Һ��V2mLδ֪Ũ�ȵ�NaOH��Һ��Ͼ��Ⱥ��������¼��Һ�¶ȣ�ʵ������ͼ2��ʾ��ʵ����ʼ�ձ���V1+V2=50mL��������������ȷ����______
A����ʵ��ʱ�����¶�Ϊ22��
B��ʵ�������ѧ�ܿ�ת��Ϊ����
CNaOH��Һ��Ũ��ԼΪ1.2mol/L
D��ʵ�������ˮ���ɵķ�Ӧ���Ƿ��ȷ�Ӧ��
��1����֪ϡǿ�ᡢϡǿ�Ӧ����1molҺ̬ˮʱ�ų�57.3kJ��������ϡ�����ϡ����������Һ����ǿ���ǿ���ϡ��Һ����Ӧ���Ȼ�ѧ����ʽΪ��NaOH��aq��+HCl ��aq���TNaCl��aq��+H2O��l����H=-57.3 kJ/mol��
�ʴ�Ϊ��NaOH��aq��+HCl��aq���TNaCl��aq��+H2O��l����H=-57.3 kJ/mol��
��2���ٵ�2������������������ɾ���������¶Ȳ�ƽ��ֵ=
=4.0C���ʴ�Ϊ��4.0��
��50mL0.50mol/L����������30mL0.50mol/L������Һ�����кͷ�Ӧ����ˮ�����ʵ���Ϊ0.05L��0.50mol/L=0.025mol����Һ������Ϊ��80ml��1g/ml=80g���¶ȱ仯��ֵΪ��T=4�棬������0.025molˮ�ų�������ΪQ=m?c?��T=80g��4.18J/��g?�棩��4.0��=1337.6J����1.3376KJ������ʵ���õ��к��ȡ�H=-
=-53.5 kJ/mol��
�ʴ�Ϊ��-53.5kJ/mol��
�ۢ����µ���10��ʱ����ʵ�飬ɢʧ������ʵ��������ֵƫС���ʢ���ȷ��
������ȡNaOH��Һ�����ʱ���Ӷ�������Һ������ƫ��ʵ��������ֵƫС���ʢ���ȷ��
�۷ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У�ɢʧ������ʵ��������ֵƫС���ʢ���ȷ��
��ʵ��ʱ�û���ͭ˿��������滷�β����������ɢʧ������ʵ��������ֵƫС���ʢ���ȷ��
��ѡ���٢ڢۢܣ�
��3��A��ʵ��ʱ���¶�ӦΪ���δ���֮ǰ���¶ȣ����ǻ����¶ȣ���A����
B�������кͷ�Ӧ����Һ�¶����ߣ�������ѧ�ܿ���ת��Ϊ���ܣ���B��ȷ��
C��ǡ�÷�Ӧʱ�μӷ�Ӧ��������Һ�������30mL����V1+V2=50mL��֪�����ĵ�����������Һ�����Ϊ20mL��
ǡ�÷�Ӧʱ����������Һ�����ʵ����ʵ�����n��
H2SO4 +2NaOH=Na2SO4+2H 2O
1 2
0.4mol/L��0.03L n
��n=0.4mol/L��0.03L��2=0.024mol������Ũ����
=1.2mol/L����C��ȷ��
D��ֻ�Ǹ÷�Ӧ���ȣ�������ˮ���ɵķ�Ӧ��һ�������Ȼ�狀�������������ķ�Ӧ������D����
��ѡBC��
�ʴ�Ϊ��NaOH��aq��+HCl��aq���TNaCl��aq��+H2O��l����H=-57.3 kJ/mol��
��2���ٵ�2������������������ɾ���������¶Ȳ�ƽ��ֵ=
| (30.1-26.1)+(29.8-25.9)+(30.4-26.3) |
| 3 |
��50mL0.50mol/L����������30mL0.50mol/L������Һ�����кͷ�Ӧ����ˮ�����ʵ���Ϊ0.05L��0.50mol/L=0.025mol����Һ������Ϊ��80ml��1g/ml=80g���¶ȱ仯��ֵΪ��T=4�棬������0.025molˮ�ų�������ΪQ=m?c?��T=80g��4.18J/��g?�棩��4.0��=1337.6J����1.3376KJ������ʵ���õ��к��ȡ�H=-
| 1.3376KJ |
| 0.025mol |
�ʴ�Ϊ��-53.5kJ/mol��
�ۢ����µ���10��ʱ����ʵ�飬ɢʧ������ʵ��������ֵƫС���ʢ���ȷ��
������ȡNaOH��Һ�����ʱ���Ӷ�������Һ������ƫ��ʵ��������ֵƫС���ʢ���ȷ��
�۷ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У�ɢʧ������ʵ��������ֵƫС���ʢ���ȷ��
��ʵ��ʱ�û���ͭ˿��������滷�β����������ɢʧ������ʵ��������ֵƫС���ʢ���ȷ��
��ѡ���٢ڢۢܣ�
��3��A��ʵ��ʱ���¶�ӦΪ���δ���֮ǰ���¶ȣ����ǻ����¶ȣ���A����
B�������кͷ�Ӧ����Һ�¶����ߣ�������ѧ�ܿ���ת��Ϊ���ܣ���B��ȷ��
C��ǡ�÷�Ӧʱ�μӷ�Ӧ��������Һ�������30mL����V1+V2=50mL��֪�����ĵ�����������Һ�����Ϊ20mL��
ǡ�÷�Ӧʱ����������Һ�����ʵ����ʵ�����n��
H2SO4 +2NaOH=Na2SO4+2H 2O
1 2
0.4mol/L��0.03L n
��n=0.4mol/L��0.03L��2=0.024mol������Ũ����
| 0.024mol |
| 0.02L |
D��ֻ�Ǹ÷�Ӧ���ȣ�������ˮ���ɵķ�Ӧ��һ�������Ȼ�狀�������������ķ�Ӧ������D����
��ѡBC��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ