��Ŀ����
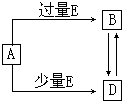 ��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮��Ĺ�ϵ��ͼ��ʾ�����ֲ�����ȥ����
��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮��Ĺ�ϵ��ͼ��ʾ�����ֲ�����ȥ������1����A��EΪ���ʣ����A���ʵ�Ԫ������Ȼ�����γɻ�������������Ԫ�أ�
��д��B���ӵĽṹʽ
����50mL4mol/L��NaOH��Һ��ͨ��1.12L����B����״��������Ӧ����Һ�����ʵ����ʵ���֮��Ϊ
��4gA��ȫȼ�շų�131.2kJ��������д����ʾA��ȼ���ȵ��Ȼ�ѧ����ʽ
��2����AΪ��������ijԪ�ص��Ȼ��0.1mol/LE��ˮ��ҺpH=13��������ɫ��Ӧ��ɫ�ʻ�ɫ��
��E�Ļ�ѧʽ
��д��ͼ��A����Һ��B����Һ�����ӷ���ʽ
��������1����A��EΪ���ʣ����A���ʵ�Ԫ������Ȼ�����γɻ�������������Ԫ�أ�ȷ��A������̼��A��������E�����E��Ӧ�������ֺ�̼������B��D�����Ʋ�E��������BΪCO2��DΪCO��
��2��0.1mol/L E��ˮ��ҺpH=13��Ϊǿ����Һ��������ɫ��Ӧ��ɫ�ʻ�ɫ������NaԪ�أ�EΪNaOH��AΪ���������е�Ԫ�����γɵ��Ȼ���벻ͬ����NaOH��Ӧ���ﲻͬ������֪AΪAlCl3��BΪNaAlO2��DΪAl��OH��3��
��2��0.1mol/L E��ˮ��ҺpH=13��Ϊǿ����Һ��������ɫ��Ӧ��ɫ�ʻ�ɫ������NaԪ�أ�EΪNaOH��AΪ���������е�Ԫ�����γɵ��Ȼ���벻ͬ����NaOH��Ӧ���ﲻͬ������֪AΪAlCl3��BΪNaAlO2��DΪAl��OH��3��
����⣺��1����A��EΪ���ʣ����A���ʵ�Ԫ������Ȼ�����γɻ�������������Ԫ�أ�ȷ��A������̼��A��������E�����E��Ӧ�������ֺ�̼������B��D�����Ʋ�E��������BΪCO2��DΪCO����
��CO2������Cԭ������ԭ��֮���γ�2�Թ��õ��Ӷԣ���ṹʽΪO=C=O��������C��Oԭ�Ӷ���8�����ȶ��ṹ��
�ʴ�Ϊ��O=C=O���ǣ�
��CO2ͨ��NaOH��Һ�з���������Ӧ��CO2+NaOH���������TNaHCO3��CO2+2NaOH���������TNa2CO3+H2O��n��NaOH��=4mol/L��0.05L=0.2mol��n��CO2��=
=0.05mol��n��CO2����n��NaOH��=0.05mol��0.2mol=1��4���ɼ�NaOH������CO2ȫ����Ӧ����Һ������ΪNa2CO3��NaOH��n��Na2CO3��=n��CO2��=0.05mol�������������غ�n��NaOH��=0.2mol-0.05mol��2=0.1mol������Һ��n��Na2CO3����n��NaOH��=0.05mol��0.1mol=1��2��
�ʴ�Ϊ��n��Na2CO3����n��NaOH��=1��2��
��1mol̼��ȫȼ�շų�������Ϊ131.2kJ��
=393.6kJ����÷�Ӧ���Ȼ�ѧ����ʽΪ��C��s��+O2��g��=CO2��g����H=-393.6kJ/mol��
�ʴ�Ϊ��C��s��+O2��g��=CO2��g����H=-393.6kJ/mol��
��2��0.1mol/L E��ˮ��ҺpH=13��Ϊǿ����Һ������ɫ��Ӧ��ɫ�ʻ�ɫ������NaԪ�أ�EΪNaOH��AΪ���������е�Ԫ�����γɵ��Ȼ���벻ͬ����NaOH��Ӧ���ﲻͬ������֪AΪAlCl3��BΪNaAlO2��DΪAl��OH��3����
��E�Ļ�ѧʽΪNaOH���������Ӽ������ۼ����ʴ�Ϊ��NaOH�����Ӽ������ۼ���
��A����Һ��B����Һ�����ӷ���ʽΪ��Al3++4OH-=AlO2-+2H2O���ʴ�Ϊ��Al3++4OH-=AlO2-+2H2O��
��CO2������Cԭ������ԭ��֮���γ�2�Թ��õ��Ӷԣ���ṹʽΪO=C=O��������C��Oԭ�Ӷ���8�����ȶ��ṹ��
�ʴ�Ϊ��O=C=O���ǣ�
��CO2ͨ��NaOH��Һ�з���������Ӧ��CO2+NaOH���������TNaHCO3��CO2+2NaOH���������TNa2CO3+H2O��n��NaOH��=4mol/L��0.05L=0.2mol��n��CO2��=
| 1.12L |
| 22.4L/mol |
�ʴ�Ϊ��n��Na2CO3����n��NaOH��=1��2��
��1mol̼��ȫȼ�շų�������Ϊ131.2kJ��
| 1mol��12g/mol |
| 4g |
�ʴ�Ϊ��C��s��+O2��g��=CO2��g����H=-393.6kJ/mol��
��2��0.1mol/L E��ˮ��ҺpH=13��Ϊǿ����Һ������ɫ��Ӧ��ɫ�ʻ�ɫ������NaԪ�أ�EΪNaOH��AΪ���������е�Ԫ�����γɵ��Ȼ���벻ͬ����NaOH��Ӧ���ﲻͬ������֪AΪAlCl3��BΪNaAlO2��DΪAl��OH��3����
��E�Ļ�ѧʽΪNaOH���������Ӽ������ۼ����ʴ�Ϊ��NaOH�����Ӽ������ۼ���
��A����Һ��B����Һ�����ӷ���ʽΪ��Al3++4OH-=AlO2-+2H2O���ʴ�Ϊ��Al3++4OH-=AlO2-+2H2O��
���������⿼��������ƶϣ��漰���ӽṹ����ѧ���㡢�Ȼ�ѧ����ʽ�����ӷ���ʽ��д����Ҫѧ����������Ԫ�ػ���������ʣ��ѶȲ���ע��Ի���֪ʶ��ȫ�����գ�

��ϰ��ϵ�д�
�����Ŀ
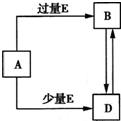 ��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮��Ĺ�ϵ��ͼ��ʾ�����ֲ�����ȥ��������˵��������ǣ�������
��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮��Ĺ�ϵ��ͼ��ʾ�����ֲ�����ȥ��������˵��������ǣ������� ��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮��Ĺ�ϵ��ͼ��ʾ�����ֲ�����ȥ����
��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮��Ĺ�ϵ��ͼ��ʾ�����ֲ�����ȥ����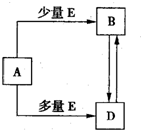 ��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮��Ĺ�ϵ��ͼ��ʾ�����ֲ�����ȥ����
��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮��Ĺ�ϵ��ͼ��ʾ�����ֲ�����ȥ����