��Ŀ����
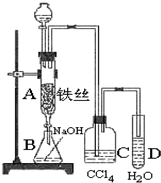
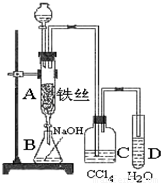
ij��ѧ����С����ͼ6-21��ʾװ����ȡ�屽�������Һ©���м��뱽��Һ�壬�ٽ����Һ�������뷴Ӧ��A(A�¶˻����ر�)�С� 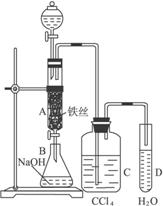
ͼ6-21
(1)д��A�з�Ӧ�Ļ�ѧ����ʽ______________________________________��
(2)�۲쵽A�е�������___________________________��
(3)ʵ�����ʱ����A�¶˵Ļ������÷�ӦҺ����B�У������Ŀ����_____________��д���йصĻ�ѧ����ʽ__________________________��
(4)C��ʢ��CCl4��������____________________________��
(5)��֤������Һ�巢������ȡ����Ӧ�������Ǽӳɷ�Ӧ�������Թ�D�м���AgNO3��Һ������������ɫ����������֤������һ����֤�ķ��������Թ�D�м���______________��������___________________________________��
���������ڱ���Һ��ķе�ϵͣ��ҷ�ӦC6H6+Br2![]() C6H5Br+HBrΪ���ȷ�Ӧ����A�й۲쵽������Ϊ����ӦҺ�У��к���ɫ�������A������HBr�л��е�Br2�ھ���CCl4ʱ�����գ����Ʊ����屽�г�����Br2��һ�����NaOH��Һ�У�������ӦBr2+2NaOH====NaBr+NaBrO+H2O����ȥ�����÷�ӦΪȡ����Ӧ�������HBr���ɣ���Ϊ�ӳɷ�Ӧ����û��HBr���ɣ���ֻ����D���Ƿ��д���H+��Br-���ɡ�
C6H5Br+HBrΪ���ȷ�Ӧ����A�й۲쵽������Ϊ����ӦҺ�У��к���ɫ�������A������HBr�л��е�Br2�ھ���CCl4ʱ�����գ����Ʊ����屽�г�����Br2��һ�����NaOH��Һ�У�������ӦBr2+2NaOH====NaBr+NaBrO+H2O����ȥ�����÷�ӦΪȡ����Ӧ�������HBr���ɣ���Ϊ�ӳɷ�Ӧ����û��HBr���ɣ���ֻ����D���Ƿ��д���H+��Br-���ɡ�
�𰸣�(1)C6H6+Br2![]() C6H5Br+HBr
C6H5Br+HBr
(2)��ӦҺ�У��к���ɫ�������A����
(3)��ȥ�����屽�е��� Br2+2NaOH====NaBr+NaBrO+H2O��3Br2+6NaOH====5NaBr+
NaBrO3+3H2O
(4)��ȥ�廯�������е�������
(5)ʯ����Һ ��Һ���ɫ

 ���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�п�ϵ�д� ���ǶԱ��Ľṹ�����ʵ���ʶ������һ�������Ĺ��̣�
���ǶԱ��Ľṹ�����ʵ���ʶ������һ�������Ĺ��̣� +Br
+Br -Br+HBr
-Br+HBr