ЬтФПФкШн
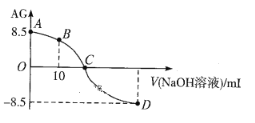
ЁОЬтФПЁПВнЫсОЇЬхЕФзщГЩПЩБэЪОЮЊH2C2O4ЁЄxH2OЃЌЮЊСЫВтЖЈxжЕЃЌНјааЯТЪіЪЕбщЃКЂйГЦШЁngВнЫсОЇЬхХфГЩ100.00mLЫЎШмвКЃЛЂкШЁ25.00 mLЫљХфжЦЕФВнЫсШмвКжУгкзЖаЮЦПжаЃЌМгЯЁСђЫсЃЌгУХЈЖШЮЊamolЁЄLЃ1ЕФKMnO4ШмвКЕЮЖЈЃЌЫљЗЂЩњЕФЗДгІЮЊЃК2KMnO4ЃЋ5H2C2O4ЃЋ3H2SO4=K2SO4ЃЋ10CO2ЁќЃЋ2MnSO4ЃЋ8H2OЁЃЗДгІЩњГЩMnSO4дкЫЎШмвКжаЛљБОЮоЩЋЃЌЪдЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉЪЕбщЬЈЩЯгавдЯТвЧЦїЃЌЪЕбщжаВЛашвЊЕФЪЧ___ЃЈЬюађКХЃЉЁЃ
a.ЭаХЬЬьЦНЃЈДјэРТыЁЂФїзгЃЉ b.ЕЮЖЈЙм c.100 mLШнСПЦП d.ЩеБ e.ТЉЖЗ f.зЖаЮЦП g.ВЃСЇАє h.вЉГз i.ЩеЦП
ЃЈ2ЃЉЪЕбщжаKMnO4ШмвКгІзАдк___ЪНЕЮЖЈЙмжаЃЌдвђЪЧ___ЁЃ
ЃЈ3ЃЉЕЮЖЈЙ§ГЬжагУШЅVmLamolЁЄLЃ1ЕФKMnO4ШмвКЃЌдђЫљХфжЦЕФВнЫсЕФЮяжЪЕФСПХЈЖШЮЊ__molЁЄLЃ1ЃЌгЩДЫПЩМЦЫуxЕФжЕЮЊ___ЁЃ
ЃЈ4ЃЉШєЕЮЖЈжеЕуЖСЪ§ЪБИЉЪгЃЌдђМЦЫуГіЕФxжЕПЩФм__ЃЈЬюЦЋДѓЁЂЦЋаЁЁЂЮогАЯьЃЉЁЃ
ЁОД№АИЁПe i Ыс KMnO4гаЧПбѕЛЏадЃЌЫќФмИЏЪДЯ№НКЃЌЙЪВЛПЩвдгУМюЪНЕЮЖЈЙмЪЂЗХ ![]()
![]() -5 ЦЋДѓ
-5 ЦЋДѓ
ЁОНтЮіЁП
ЃЈ1ЃЉЪЕбщжаашвЊХфжЦЮяжЪЕФСПХЈЖШКЭЕЮЖЈЪЕбщЃЌХфжЦВнЫсЕФЮяжЪЕФСПХЈЖШашвЊвЧЦїЃКЭаХЬЬьЦНЁЂвЉГзЁЂЩеБЁЂВЃСЇАєЁЂ100mLШнСПЦПЁЂНКЭЗЕЮЙмЃЌЕЮЖЈЪЕбщЪБашвЊвЧЦїЃКЫсЪНЁЂМюЪНЕЮЖЈЙмЁЂзЖаЮЦПЁЂЬњМмЬЈЃЌвђДЫВЛашвЊЕФвЧЦїЪЧЩеЦПКЭТЉЖЗЃЌЙЪeiЗћКЯЬтвтЃЛ
Д№АИЮЊeiЃЛ
ЃЈ2ЃЉKMnO4ОпгаЧПбѕЛЏадЃЌФмбѕЛЏМюЪНЕЮЖЈЙмЯТЖЫЕФЯ№НКЙмЃЌвђДЫЪЂЗХKMnO4ШмвКЕФЕЮЖЈЙмЮЊЫсЪНЕЮЖЈЙмЃЛ
Д№АИЮЊЫсЪНЃЛKMnO4гаЧПбѕЛЏадЃЌЫќФмИЏЪДЯ№НКЃЌЙЪВЛПЩвдгУМюЪНЕЮЖЈЙмЪЂЗХЃЛ
ЃЈ3ЃЉИљОнЗДгІЗНГЬЪНЃЌЫљХфЕФВнЫсЕФЮяжЪЕФСПХЈЖШЮЊ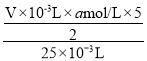 =
=![]() molЁЄLЃ1ЃЛ100.00mLШмвКжаВнЫсЕФЮяжЪЕФСПЮЊ100ЁС10Ѓ3LЁС
molЁЄLЃ1ЃЛ100.00mLШмвКжаВнЫсЕФЮяжЪЕФСПЮЊ100ЁС10Ѓ3LЁС![]() molЁЄLЃ1=0.01VamolЃЌВнЫсОЇЬхЕФЮяжЪЕФСПЮЊ0.01VamolЁЄLЃ1ЃЌжЪСПЮЊngЃЌдђга0.01VamolЁС(90ЃЋ18x)gЁЄmolЃ1=nЃЌНтЕУx=
molЁЄLЃ1=0.01VamolЃЌВнЫсОЇЬхЕФЮяжЪЕФСПЮЊ0.01VamolЁЄLЃ1ЃЌжЪСПЮЊngЃЌдђга0.01VamolЁС(90ЃЋ18x)gЁЄmolЃ1=nЃЌНтЕУx=![]() -5 ЃЛ
-5 ЃЛ
Д№АИЮЊ![]() ЃЛ
ЃЛ![]() -5ЃЛ
-5ЃЛ
ЃЈ4ЃЉЕЮЖЈжеЕуЪБИЉЪгЖСЪ§ЃЌVЦЋаЁЃЌИљОнx=![]() -5ЃЌдђxЦЋДѓЃЛ
-5ЃЌдђxЦЋДѓЃЛ
Д№АИЮЊЦЋДѓЁЃ

 дФЖСПьГЕЯЕСаД№АИ
дФЖСПьГЕЯЕСаД№АИЁОЬтФПЁПФГГЧЪаЖдДѓЦјНјааМрВтЃЌЗЂЯжИУЪаЪзвЊЮлШОЮяЮЊПЩЮќШыПХСЃЮяPM2.5(жБОЖаЁгкЕШгк2.5ІЬmЕФаќИЁПХСЃЮя)ЃЌЦфжївЊРДдДЮЊШМУКЁЂЛњЖЏГЕЮВЦјЕШЁЃвђДЫЃЌЖдPM2.5ЁЂSO2ЁЂNOxЕШНјаабаОПОпгаживЊвтвхЁЃЧыЛиД№ЯТСаЮЪЬтЃК
(1)PM2.5ЗжЩЂдкПеЦјжааЮГЩЕФЗжЩЂЯЕ________(ЬюЁАЪєгкЁБЛђЁАВЛЪєгкЁБ)НКЬхЁЃ
(2)НЋPM2.5бљБОгУеєСѓЫЎДІРэжЦГЩД§ВтЪдбљЁЃШєВтЕУИУЪдбљЫљКЌЫЎШмадЮоЛњРызгЕФЛЏбЇзщЗжМАЦфЦНОљХЈЖШШчЯТБэЃК
Рызг | KЃЋ | NaЃЋ | NH4+ | SO42- | NO3- | ClЃ |
ХЈЖШ/ molЁЄLЃ1 | 4ЁС10Ѓ6 | 6ЁС10Ѓ6 | 2ЁС10Ѓ5 | 4ЁС10Ѓ5 | 3ЁС10Ѓ5 | 2ЁС10Ѓ5 |
ИљОнБэжаЪ§ОнХаЖЯД§ВтЪдбљЮЊ________(ЬюЁАЫсЁБЛђЁАМюЁБ)адЃЌБэЪОИУЪдбљЫсМюадЕФc(HЃЋ)Лђc(OHЃ)ЃН________molЁЄLЃ1ЁЃ
(3)ЮЊМѕЩйSO2ЕФХХЗХЃЌПЩгУФГаЉШмвКЯДЕгКЌSO2ЕФбЬЦјЁЃвдЯТЮяжЪПЩзіЯДЕгМСЕФЪЧ__________________________(ЬюзжФИ)ЁЃ
a.Ca(OH)2ЁЁЁЁb.Na2CO3ЁЁЁЁc.CaCl2ЁЁЁЁd.NaHSO3
(4)ЦћГЕЮВЦјжаNOxКЭCOЕФЩњГЩМАзЊЛЏЁЃ
ЂйЦћГЕЦєЖЏКѓЃЌЦћИзЮТЖШдНИпЃЌЕЅЮЛЪБМфФкNOХХЗХСПдНДѓЃЌаДГіЦћИзжаЩњГЩNOЕФЛЏбЇЗНГЬЪНЃК_____________________________ЁЃ
ЂкЦћГЕШМгЭВЛЭъШЋШМЩеЪБВњЩњCOЃЌФПЧАЃЌдкЦћГЕЮВЦјЯЕЭГжазАжУДпЛЏзЊЛЏЦїПЩМѕЩйCOКЭNOЕФЮлШОЃЌЦфЛЏбЇЗДгІЗНГЬЪНЮЊ__________________________ЁЃ
ЁОЬтФПЁПдк3ИіЬхЛ§ОљЮЊ4.0 LЕФКуШнУмБеШнЦїжаЃЌЗДгІ CO2(g)ЃЋC(s)![]() 2CO(g) ІЄH > 0ЃЌЗжБ№дквЛЖЈЮТЖШЯТДяЕНЛЏбЇЦНКтзДЬЌЁЃЯТСаЫЕЗЈе§ШЗЕФЪЧ
2CO(g) ІЄH > 0ЃЌЗжБ№дквЛЖЈЮТЖШЯТДяЕНЛЏбЇЦНКтзДЬЌЁЃЯТСаЫЕЗЈе§ШЗЕФЪЧ
ШнЦї | ЮТЖШ/K | Ц№ЪМЪБЮяжЪЕФСП/mol | ЦНКтЪБЮяжЪЕФСП/mol | ||
n(CO2) | n(C) | n(CO) | n(CO) | ||
I | 977 | 0.56 | 1.12 | 0 | 0.8 |
II | 977 | 1.12 | 1.12 | 0 | x |
III | 1250 | 0 | 0 | 1.12 | y |
A.977 KЃЌИУЗДгІЕФЛЏбЇЦНКтГЃЪ§жЕЮЊ4
B.ДяЕНЦНКтЪБЃЌЯђШнЦїIжадіМгCЕФСПЃЌЦНКте§ЯђвЦЖЏ
C.ДяЕНЦНКтЪБЃЌШнЦїIжаCO2ЕФзЊЛЏТЪБШШнЦїIIжаЕФДѓ
D.ДяЕНЦНКтЪБЃЌШнЦїIIIжаЕФCOЕФзЊЛЏТЪДѓгк28.6%