��Ŀ����
����Ŀ�������ģ��������и߷��ӻ�����ش�����
��1���ϳɾ۱�ϩ���Ƶĵ���Ľṹ��ʽ��________����Ӧ������____________��
��2���ٺϳɵ��������ֵ��壬�����ܺ�NaHCO3��Ӧ�ĵ���Ľṹ��ʽ��_____����˴Ź������ķ��������_____��
�ںϳɵ��ڵĻ�ѧ��Ӧ����ʽ_________________________________________��
��3���ٺϳɷ�ȩ��֬��Ҫ���ֵ��壬�������ֵ�������Է�����Ӧ���Լ���_______��ѡ����ĸ����
a��H2 b��Na c��Na2CO3 d�����Ը������
�����ж������ͷ�ȩ��֬���Ʊ������ʵ�˵���У���ȷ����____ ��ѡ����ĸ����
a�������������Ʊ� b�������������Ʊ�
c�����������Ҵ� d���������κ��ܼ�
��![]() ����ȩ������������Ҳ���Է����������Ʊ���ȩ��֬�ķ�Ӧ���˷�Ӧ�Ļ�ѧ����ʽ��__________________________________________________��
����ȩ������������Ҳ���Է����������Ʊ���ȩ��֬�ķ�Ӧ���˷�Ӧ�Ļ�ѧ����ʽ��__________________________________________________��
���𰸡� ![]() �Ӿ۷�Ӧ
�Ӿ۷�Ӧ ![]() 1:2����2: 1�� n
1:2����2: 1�� n![]() +nHOCH2CH2OH
+nHOCH2CH2OH![]()
![]() +(2n+1)H2O ad a��c
+(2n+1)H2O ad a��c 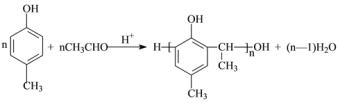
��������
��1���ϳɾ۱�ϩ���Ƶĵ���Ľṹ��ʽ��![]() ���÷�Ӧ�ǼӾ۷�Ӧ����2���ٵ���
���÷�Ӧ�ǼӾ۷�Ӧ����2���ٵ���![]() ���ɶԱ���������Ҷ������۶��ɣ�
���ɶԱ���������Ҷ������۶��ɣ�![]() �ܺ�NaHCO3��Ӧ���˴Ź������ķ��������1��2���ڶԱ���������Ҷ����������۷�Ӧ�ϳɵ��ڣ���Ӧ�Ļ�ѧ��Ӧ����ʽΪn
�ܺ�NaHCO3��Ӧ���˴Ź������ķ��������1��2���ڶԱ���������Ҷ����������۷�Ӧ�ϳɵ��ڣ���Ӧ�Ļ�ѧ��Ӧ����ʽΪn![]() +nHOCH2CH2OH
+nHOCH2CH2OH![]()
![]() +(2n+1)H2O����3���ٺϳɷ�ȩ��֬
+(2n+1)H2O����3���ٺϳɷ�ȩ��֬ �ĵ����DZ��Ӻͼ�ȩ�����Ӻͼ�ȩ�������������ӳɷ�Ӧ�������뱽�ӷ�Ӧ���ɱ����ƺ�������̼�������뱽�ӷ�Ӧ���ɱ����ƺ�̼�����ƣ����Ӻͼ�ȩ���ܱ����Ը�������������ʴ�Ϊ��ad���ڱ������ȩ�����������¾ۺ��������ͷ�ȩ��֬�����������¾ۺ��������ͷ�ȩ��֬�����ͷ�ȩ��֬���������Ҵ����ʴ�Ϊ��ac����
�ĵ����DZ��Ӻͼ�ȩ�����Ӻͼ�ȩ�������������ӳɷ�Ӧ�������뱽�ӷ�Ӧ���ɱ����ƺ�������̼�������뱽�ӷ�Ӧ���ɱ����ƺ�̼�����ƣ����Ӻͼ�ȩ���ܱ����Ը�������������ʴ�Ϊ��ad���ڱ������ȩ�����������¾ۺ��������ͷ�ȩ��֬�����������¾ۺ��������ͷ�ȩ��֬�����ͷ�ȩ��֬���������Ҵ����ʴ�Ϊ��ac����![]() �ͺ���ȩ��Ӧ�ķ���ʽ���Ա�ʾΪ��
�ͺ���ȩ��Ӧ�ķ���ʽ���Ա�ʾΪ�� ��
��

����Ŀ����֪��CH3CH2CH2CH2OH![]() CH3CH2CH2CHO��������ͼװ�����������ϳ�����ȩ��
CH3CH2CH2CHO��������ͼװ�����������ϳ�����ȩ��
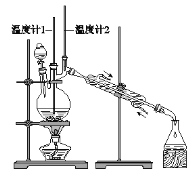
����������£�
���� | �е�/�� | �ܶ�/(g��cm��3) | ˮ���ܽ��� |
������ | 117.2 | 0.8109 | �� |
����ȩ | 75.7 | 0.8017 | �� |
��1��ʵ������У��������������������ữ��Na2Cr2O7������ԭ��___________������ҩƷʱ��Ӧ��_________________��μ���____________________�С�
��2��Ϊ��ʵ��İ�ȫ�������ڷ�Ӧ�����м���____________________��
��3����Ӧ�������¶ȼ�1ʾ��Ϊ_____�棬�¶ȼ�2ʾ����_____ʱ���ռ����
��4����Ӧ������Ϊ������ȩ��ˮ��ֿ���������ﵹ���Һ©���У�������ȩ�ӷ�Һ©��_____�ڷֳ���
��5�����õĴ�����ȩ�м���___________�����������Ƿ�������ˮ��








