��Ŀ����
18�� һ���¶��£�ij1L����̶����ܱ������У�X��Y��Z������������ʵ�����ʱ��仯��������ͼ��ʾ����ش��������⣺
һ���¶��£�ij1L����̶����ܱ������У�X��Y��Z������������ʵ�����ʱ��仯��������ͼ��ʾ����ش��������⣺��1���÷�Ӧ����������Z��
��2��0��10sʱ��ƽ����Ӧ����v��Z��=0.158mol/��L•s����
��3��0��10sʱ��ƽ����Ӧ����v��X��=���������������=����v��Y����
��4���÷�Ӧ�Ļ�ѧ����ʽΪX+Y?2Z
��5��10s������Ϊ�ﵽƽ��״̬�ı�־�Ǣڣ���ѡ������ţ�
�ٵ�λʱ��������1molX��ͬʱ����1molY
�ڵ�λʱ��������1molX��ͬʱ����2molZ
�ۻ��������ܶȲ��ٸı�
�������е�ѹǿ����ʱ��ı仯���ı䣮
���� ��1�����ݷ�Ӧ�����������ʵ������ӣ���Ӧ�����ʵ�����С�жϣ�
��2������v=$\frac{��c}{��t}$���㻯ѧ��Ӧ���ʣ�
��3������v=$\frac{��c}{��t}$����X��Y�Ļ�ѧ��Ӧ���ʲ��Ƚϣ�
��4�����ݻ�ѧ��Ӧ�и����ʵ����ʵ����仯���뻯ѧ������֮�ȳ�������д��ѧ����ʽ��
��5������ƽ��״̬���淴Ӧ������ȣ�����ֵ�Ũ�Ȼ�������������Լ���Ӧ������ijЩ�����������ı仯�жϣ�
��� �⣺��1������ͼ��֪��Z���ʵ����ɿ�ʼ��0mol�仯10sʱ��1.58mol������ZΪ�����
�ʴ�Ϊ��Z��
��2����Ӧ��ʼ��10s����Z��ʾ�ķ�Ӧ����Ϊ��v=$\frac{��c}{��t}$=$\frac{\frac{1.58mol}{1L}}{10s}$=0.158mol/��L•s����
�ʴ�Ϊ��0.158mol/��L•s����
��3������ͼ�е����ݿ�֪��v��X��=$\frac{\frac{1.20-0.41}{1}}{10}$mol/��L•s��=0.079mol/��L•s����v��Y��=$\frac{\frac{1.00-0.21}{1}}{10}$mol/��L•s��=0.079mol/��L•s��������v��X��=v��Y����
�ʴ�Ϊ��=��
��4����ͼ����Կ���X��Y�����ʵ�����С��Z�����ʵ������࣬��X��YΪ��Ӧ�ZΪ�����
��ѧ��Ӧ�и����ʵ����ʵ����仯���뻯ѧ������֮�ȳ����ȣ�
����Y��X��Z=��1.20mol-0.41mol������1.0mol-0.21mol����1.58mol=1��1��2����Ӧ�Ļ�ѧ����ʽΪX+Y?2Z��
�ʴ�Ϊ��X+Y?2Z��
��5�����ݷ�Ӧ��X+Y?2Z��
�ٵ�λʱ��������1molX��ͬʱ����1molY������ָ�淴Ӧ���ʣ����ܷ�ӳ�����淴Ӧ���� �Ĺ�ϵ���ʴ���
�ڵ�λʱ��������1molX��ͬʱ����2molZ��˵�����淴Ӧ������ȣ����Է�Ӧ����ƽ��״̬������ȷ��
�۸÷�Ӧ�ں��������½��У���Ӧǰ��������������䣬�����ܶȲ��淴Ӧ�Ľ��ж��仯�����Ի��������ܶȲ��ٸı䣬����˵����Ӧ�Ƿ���ƽ��״̬���ʴ���
�ܸ÷�ӦΪ��Ӧǰ��������������ķ�Ӧ�������е�ѹǿ���淴Ӧ�Ľ��ж��仯�������е�ѹǿ����ʱ��ı仯���ı䣬����˵����Ӧ�Ƿ���ƽ��״̬���ʴ���
��ѡ�ڣ�
���� ���⿼�����ʵ�����ʱ��ı仯���ߣ��Լ���ѧƽ��ļ��㣬��Ŀ�Ѷ��еȣ�ע����ջ�ѧ����ʽ���жϷ�����

 ��У����ϵ�д�
��У����ϵ�д�| A�� | NaCl | B�� | CO2 | C�� | C12H22O11�����ǣ� | D�� | Al |
���Թ� ���ձ� �۾ƾ��� ���Թܼ� ��ʯ������
| A�� | �٢ڢ� | B�� | �ڢۢ� | C�� | �٢ۢ� | D�� | �ڢۢ� |
| A�� | ��ɫ��Һ�м���AgNO3��Һ�ð�ɫ��������ϡ�����ʧ�����ܴ���Cl-��SO42- | |
| B�� | ��ɫ��Һ�еμ�BaCl2��Һ�ð�ɫ��������ϡ����ܽ⣬��һ������SO42- | |
| C�� | ��CCl4��ȡ��ˮ�еĵ⣬�²���Ϻ�ɫ | |
| D�� | �������ᣬ���ɵ�������ʹ����ʯ��ˮ����ǣ���ԭ��Һ��һ���д���CO32- |
| A�� | HBr��CH3COONa��BaSO4 | B�� | NH4Cl��H2O��Na2S | ||
| C�� | NaOH��Ca��OH��2��NH3•H2O | D�� | HClO��NaF��Ba��OH��2 |
 ��
�� ��Ԫ��D��Ԫ�����ڱ��е�λ���ǵ�������VIIA�壮
��Ԫ��D��Ԫ�����ڱ��е�λ���ǵ�������VIIA�壮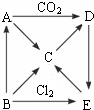 A��B��C��D��E������ɫ��Ӧ���Ի�ɫ����Щ������ʵ����ͼ��ʾ��ת����ϵ������AΪ����ɫ���壬CΪǿ�
A��B��C��D��E������ɫ��Ӧ���Ի�ɫ����Щ������ʵ����ͼ��ʾ��ת����ϵ������AΪ����ɫ���壬CΪǿ�