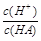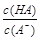��Ŀ����
��10�֣��������ԭ�����ش����и�С�⣺
��1��25��ʱ��ijFeCl3��Һ��pH=2������ˮ�����������c(OH-)= �������ӷ���ʽ��ʾFeCl3��Һ���ھ�ˮ��ԭ�� ��
��2����֪NaHSO4��ˮ�еĵ��뷽��ʽNaHSO4��Na+��H+��SO42-��
��NaHSO4��Һ��c(H+) c(OH-)��c(SO42-)���>������������<����ͬ��������������������������Һ��ȡ���ᱵ������Һ��SO42-��ȫ��������Ӧ����Һ��pH 7��
��3����0.02mol/LNa2SO4��Һ��ijŨ��BaCl2��Һ�������ϣ�������BaSO4��������ԭBaCl2��Һ����СŨ��Ϊ ��(��֪Ksp(BaSO4)��1.1��10-10)
��4��д�����з�Ӧ�Ļ�ѧ����ʽ��
����������Ӧ �� �Ҵ��Ĵ�������Ӧ ��
��1��25��ʱ��ijFeCl3��Һ��pH=2������ˮ�����������c(OH-)= �������ӷ���ʽ��ʾFeCl3��Һ���ھ�ˮ��ԭ�� ��
��2����֪NaHSO4��ˮ�еĵ��뷽��ʽNaHSO4��Na+��H+��SO42-��
��NaHSO4��Һ��c(H+) c(OH-)��c(SO42-)���>������������<����ͬ��������������������������Һ��ȡ���ᱵ������Һ��SO42-��ȫ��������Ӧ����Һ��pH 7��
��3����0.02mol/LNa2SO4��Һ��ijŨ��BaCl2��Һ�������ϣ�������BaSO4��������ԭBaCl2��Һ����СŨ��Ϊ ��(��֪Ksp(BaSO4)��1.1��10-10)
��4��д�����з�Ӧ�Ļ�ѧ����ʽ��
����������Ӧ �� �Ҵ��Ĵ�������Ӧ ��
��10�֣�
��1��10-2mol/L ��1�֣� Fe3++3H2O Fe(OH)3(����)+3H+ ��1�֣�
Fe(OH)3(����)+3H+ ��1�֣�
��2�� =��1�֣� >��1�֣�
��3�� 2.2��10-8mol/L��2�֣� ��4��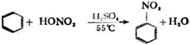 2 C2H5OH + O2
2 C2H5OH + O2  2 CH3CHO + 2H2O����2�֣�
2 CH3CHO + 2H2O����2�֣�
��1��10-2mol/L ��1�֣� Fe3++3H2O
 Fe(OH)3(����)+3H+ ��1�֣�
Fe(OH)3(����)+3H+ ��1�֣� ��2�� =��1�֣� >��1�֣�
��3�� 2.2��10-8mol/L��2�֣� ��4��
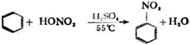 2 C2H5OH + O2
2 C2H5OH + O2 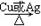 2 CH3CHO + 2H2O����2�֣�
2 CH3CHO + 2H2O����2�֣������������1��FeCl3��ǿ�������Σ�����ˮ����ˮ������ԣ���Fe3++3H2O
 Fe(OH)3(����)+3H+��H+ȫ������ˮ�������ɵģ�pH=2��c(H+)=10-2mol/L��c(OH-)ˮ= c(H+)ˮ=10-2mol/L���������������ӣ��ﵽ��ˮ��Ŀ�ġ���2����NaHSO4��Һ�е�H+����ˮ�ĵ����NaHSO4��ˮ�еĵ��룬 OH-����ˮ�ĵ��룬c(OH-)ˮ������ˮ�����c(H+)ˮ��c(SO42-)������NaHSO4��ˮ�еĵ����H+��c(H+)�����c(H+)=c(OH-)��c(SO42-)������Һ��SO42-��ȫ������NaHSO4+Ba(OH)2= BaSO4��+H2O+NaOH����Ӧ������ΪNaOH���ʼ��ԣ�pH>7����3��������ԭBaCl2��Һ����СŨ��Ϊx,Ksp(BaSO4)= c(Ba2+)* c(SO42-),1.1��10-10=(0.02/2)*(x/2),���x=2.2��10-8mol/L��
Fe(OH)3(����)+3H+��H+ȫ������ˮ�������ɵģ�pH=2��c(H+)=10-2mol/L��c(OH-)ˮ= c(H+)ˮ=10-2mol/L���������������ӣ��ﵽ��ˮ��Ŀ�ġ���2����NaHSO4��Һ�е�H+����ˮ�ĵ����NaHSO4��ˮ�еĵ��룬 OH-����ˮ�ĵ��룬c(OH-)ˮ������ˮ�����c(H+)ˮ��c(SO42-)������NaHSO4��ˮ�еĵ����H+��c(H+)�����c(H+)=c(OH-)��c(SO42-)������Һ��SO42-��ȫ������NaHSO4+Ba(OH)2= BaSO4��+H2O+NaOH����Ӧ������ΪNaOH���ʼ��ԣ�pH>7����3��������ԭBaCl2��Һ����СŨ��Ϊx,Ksp(BaSO4)= c(Ba2+)* c(SO42-),1.1��10-10=(0.02/2)*(x/2),���x=2.2��10-8mol/L������������֪ʶ��Ƚϴ�˼������������Һ�У�������Ũ�ȳ������������ӵ�Ũ�ȵ���ˮ�����ӻ�����������ˮ��������������ӵ�Ũ�ȵ�����ˮ����������ӵ�Ũ�ȡ�NaHSO4��ˮ������ȫ���롣Ksp�ļ��㣬��������AnBm��s��="n" Am+(aq)+ mBn-(aq), �ܶȻ�(Ksp)=(C(Am+) )^n ( C(Bn-))^m��

��ϰ��ϵ�д�
�����Ŀ
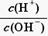 ��1010����ش��������⣺
��1010����ش��������⣺