��Ŀ����
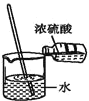
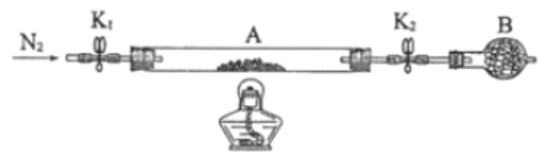
����Ŀ����������(CH3COOOH)��һ�ֳ��õ���������������ˮ���ӷ�������������ֽ⡣���Ʊ�ԭ��Ϊ��![]() ��H<0��ͬʱ�������ᶡ����ˮ�γɹ�����е�90.7�棩��ʱ�����ˮ������߲��ʡ�ʵ��װ����ͼ��
��H<0��ͬʱ�������ᶡ����ˮ�γɹ�����е�90.7�棩��ʱ�����ˮ������߲��ʡ�ʵ��װ����ͼ��

��1���������ᱣ��ʱӦע��______________�����ţ���
A���ܹ�B������C���ܱ�D����������
��2������a������Ϊ______________������ʢ�ŵ��Լ�Ϊ______________������ᡱ��˫��ˮ������
��3��Ϊ���ٷ�Ӧƿ�����ᶡ������ģ���Ӧ��ʼǰ������ˮ��������Ӧ���еIJ�����______________��
��4����Ӧ��ϵ���ü�ѹ��Ŀ����____��
��5���������ᣨ��������H2O2���ʣ��ĺ����ⶨ������ͼ��
![]()
���ж�H2O2ǡ�ó�����ʵ��������______________��
�ڹ������ᱻFe2+��ԭ����ԭ����֮һΪ���ᣬ�����ӷ���ʽΪ______________
������Ʒ���ΪVomL������c1mol��L-1FeSO4��ҺV1mL������c2mol��L-1K2Cr2O7��ҺV2mL����������Ậ��Ϊ______________g.L-l��
���𰸡�ABC ��ѹ��Һ©�� ˫��ˮ ����ˮ�������м������ᶡ�� ��ѹ���Խ�������ķе㣬��ֹ�¶ȹ��ߣ���������ֽ� ���������һ�θ��������Һʱ����Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ CH3COOOH+ 2Fe2++2H+=CH3COOH+2Fe3++H2O ![]()
��������
������������ڷ�Ӧƿ�з�Ӧ���ɹ��������ˮ������ˮ�����ᶡ���ķе�����γɹ��������ˮ��ʹ��Ӧ���ƣ���߲��ʡ�������ˮ���������ᶡ������������������������Һ̬��ˮ�����ᶡ�������ܣ���ˮ���ܶȴ����²㣬�ϲ����ᶡ�����˺������Ӧƿѭ����������ᶡ���������ʡ�
(1)��������������ˮ���ӷ�������������ֽ⣬��Ӧ���ܹ⡢���¡��ܱձ��棬�ʴ�Ϊ��ABC��
(2)����aΪ��ѹ��Һ©������˫��ˮ�ֽ⣬����ʢ��˫��ˮ����Ҫʱ�μ�˫��ˮ���ʴ�Ϊ����ѹ��Һ©����˫��ˮ��
(3)��Ӧ��ʼǰ������ˮ�������м������ᶡ�����������ᶡ���ɿ��ٻ�����Ӧƿ���Ӷ����ٷ�Ӧƿ�����ᶡ������ģ��ʴ�Ϊ������ˮ�������м������ᶡ����
(4)����������������ֽ⣬��ѹ���Խ�������ķе㣬�Ӷ�����Ϊ���ͷ�Ӧƿ���¶ȣ���ֹ�¶ȹ��ߣ���������ֽ⣬�ʴ�Ϊ����ѹ���Խ�������ķе㣬��ֹ�¶ȹ��ߣ���������ֽ⣻
(5)�ٸ��������ҺΪ�Ϻ�ɫ����������û����֮ǰ���Ӹ�����أ���������Ϻ�ɫ����ȥ�������������ѳ������ٵ��������أ���Һ����Ϊ�Ϻ�ɫ���ʴ�Ϊ�����������һ�θ��������Һʱ����Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ��
��Fe2+����ԭ������������ΪFe3+����������Ϊ����������ԭ����Ϊ���ᣬ���ԭ���غ㡢��ʧ�����غ�ɵ����ӷ���ʽΪ��CH3COOOH+ 2Fe2++2H+=CH3COOH+2Fe3++H2O���ʴ�Ϊ��CH3COOOH+ 2Fe2++2H+=CH3COOH+2Fe3++H2O��
��Fe2+�����ʵ���=c1V1��10-3mol��n(K2Cr2O7)=c2V2��10-3mol�����ݵ�ʧ�����غ��У�K2Cr2O7��2Cr3+��6e-��6Fe2+��6Fe3+����ÿ1mol K2Cr2O7��6mol Fe2+ǡ����ȫ��Ӧ�����ԣ���c2V2��10-3mol K2Cr2O7��Ӧ��Fe2+�����ʵ���= 6c2V2��10-3mol����ô����������ᷴӦ��Fe2+�����ʵ���= c1V1��10-3mol-6c2V2��10-3mol������CH3COOOH+ 2Fe2++2H+=CH3COOH+2Fe3++H2O�ɵã�CH3COOOH�����ʵ���=![]() ��CH3COOOH������=
��CH3COOOH������=![]() ��76g/mol=
��76g/mol=![]() ����������Ậ��=
����������Ậ��=![]() =
=![]() g��L-l���ʴ�Ϊ��
g��L-l���ʴ�Ϊ��![]() ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ������ͼװ�ý���ʵ�飬�����ƶ���ȷ����
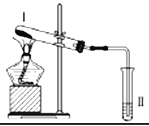
ѡ�� | I���Լ� | II���Լ������� | �ƶ� |
A | Ϳ��ʯ���͵����Ƭ | ���Ը��������Һ��ɫ | ʯ���ͷ����˻�ѧ�仯 |
B | �������� | Ʒ����Һ��ɫ | FeSO4�ֽ�����FeO��SO2 |
C | �Ȼ�� | ��̪��Һ�����ɫ | �Ȼ���ȶ� |
D | ������ˮ���� | ����ˮð�� | ������ˮ���������˷�Ӧ |
A.AB.BC.CD.D
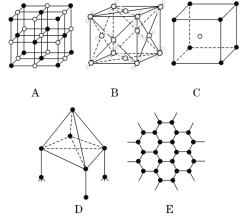
����Ŀ���±�ΪԪ�����ڱ���һ���֣�a��b��c��Ϊ����Ԫ�ء��ش��������⣺
a | |||||||||||||||||
b | c | d | e | ||||||||||||||
f | g | ||||||||||||||||
h | |||||||||||||||||
(1)�뻭������Ԫ��h��ԭ�ӽṹʾ��ͼ_______��Ԫ��hλ��Ԫ�����ڱ��ĵ�_____����
(2)e��g����Ԫ�ص���̬�⻯���и��ȶ�����_______��д��ѧʽ����
(3)b2a2���ӵĵ���ʽ��________ ���÷����д��ڵ������������ĸ�����Ϊ_______��
(4)c��d����Ԫ���е�һ�����ܽϴ����_________��дԪ�ط��ţ���
(5)f��g����Ԫ���γɵĻ�������______���������ӻ������������ۻ�����������a2d������_________���ӣ���Է��ӻ��߷Ǽ��Է��ӣ���