��Ŀ����
��������Ҫ������Դ��������Ϊ�������õĹؼ��������ǵ�ǰ��ע���ȵ�֮һ��
��1�� ���淴ӦN2+3H2 2NH3�ǹ�ҵ�Ϻϳɰ�����Ҫ��Ӧ
2NH3�ǹ�ҵ�Ϻϳɰ�����Ҫ��Ӧ
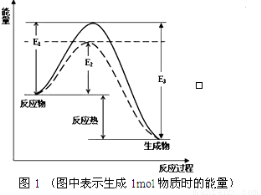
����ͼ1��д���ϳɰ����Ȼ�ѧ����ʽ��_______________��������E1��E2��E3��ʾ����
��2��LiAlH4��һ����Ҫ�Ĵ������壬����ˮ��Ӧ�ﵽLiAlO2���������÷�Ӧ����1mol LiAlH4ʱת�Ƶĵ�����ĿΪ__________��
��3��������Ƿdz���ǰ;�Ĵ�����ϣ����������м����м���ʱ�ɵõ�����ﮣ�LiNH2�����䷴Ӧ�Ļ�ѧ����ʽΪ��Li3N+2H2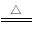 LiNH2+2LiH����������Ϊ_________���ѧʽ��,��270��ʱ���÷�Ӧ���������ų�H2���������﮿���Ϊ������ϣ������������ɴ�Li3N������_________%����ȷ��0.1����
LiNH2+2LiH����������Ϊ_________���ѧʽ��,��270��ʱ���÷�Ӧ���������ų�H2���������﮿���Ϊ������ϣ������������ɴ�Li3N������_________%����ȷ��0.1����
��4��LiFePO4��������Ӷ������������ص����Ƴ�Ϊ���˻���ɫ��Դ���³裬��֪��طŵ�ʱ�ܷ�ӦʽΪFePO4+Li�TLiFePO4 �����������ӦΪ____________________��
��5��һ�������£���ͼ��ʾװ�ÿ�ʵ���л���ĵ绯ѧ���⣨���������л����

�ٵ����е����ƶ�����Ϊ____________������A��D��ʾ��
������Ŀ�����ĵ缫��ӦʽΪ__________________________��
�۸ô���װ�õĵ���Ч�� ��__________________________��
��__________________________��
�� ��
�� ��100%������������С�����1λ��
��100%������������С�����1λ��


 �������������Һ��һ�����Դ����������������( )
�������������Һ��һ�����Դ����������������( )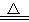 CuSO4��s��+5H2O��l������ЧӦΪ��H3���������ж���ȷ���ǣ� ��
CuSO4��s��+5H2O��l������ЧӦΪ��H3���������ж���ȷ���ǣ� ��
 ���пհף�
���пհף� CH3OH��g����
CH3OH��g����
 Ϊ0.1NA
Ϊ0.1NA
 �����ӳɷ�Ӧʱ����������5 mol H2
�����ӳɷ�Ӧʱ����������5 mol H2
 )���ᣬ���и�ѡ�������ĵ�
)���ᣬ���и�ѡ�������ĵ� ��������ʵ�����ȷ����(��λ��mol)( )
��������ʵ�����ȷ����(��λ��mol)( )